Research at Hughes Spalding. Updated Review and Approval Process
Navigate the research review and approval process efficiently as a busy clinician investigator at Hughes Spalding with insights on key responsibilities, approval levels, and contact information of relevant personnel. From pre-award tasks like facilitating reviews and gaining approvals to post-award
3 views • 6 slides
ETHICS APPLICATION 101
Overview of the ethics approval process for health research, emphasizing the importance of conducting research ethically to benefit participants and minimize risks. Explains the role of ethics committees in evaluating and minimizing potential risks. Describes the establishment of the Health Research
0 views • 21 slides
Insider Tips for Getting Your Overseas Education Loan Approved Quickly
For swift approval of your overseas education loan, research lenders thoroughly and compile a robust application. Consider a co-signer to bolster your credibility, showcase financial stability, and maintain clear communication with lenders. These insider tips streamline the loan approval process. To
1 views • 7 slides
Progress Update on S-124 Development and Approval Process
The content provides a detailed agenda for a meeting in Monaco, discussing the progress and development story of S-124, including the approval of Edition 1.0.0. It covers key topics such as the Feature Catalog, Guidance Documentation, Validation, and implementing S-124/S-412 into the GMDSS. The deve
0 views • 18 slides
Unit Operations Approval Protocol Summary
This document outlines the approval process for unit operations categorized by size and risk level, covering factors such as mitigated risk assessment, resource requirements, and operational specifics. It delineates three levels of operations based on risk assessment criteria and the involvement of
1 views • 12 slides
Understanding CMS IT Governance Training Life Cycle ID (LCID)
CMS IT Governance Training Life Cycle ID (LCID) is a crucial aspect of IT project management within the CMS organization, ensuring proper approval and tracking of IT activities. This ID is not a funding approval but signifies evaluation for feasibility, standards, and cost-effectiveness. It is essen
0 views • 11 slides
Understanding Regulatory Requirements of Drugs and Pharmaceuticals
Drug regulation involves controlling drug use through international agreement authorities like the FDA, EMA, and PMDA. The FDA plays a crucial role in drug evaluation and research, biologic evaluation, devices, and food safety. There are various types of applications for drug approval, along with a
0 views • 28 slides
IAEC Approval Presentation: Drug Discovery Study Overview
This presentation template provides guidelines for scholars to prepare and present their research study to the IAEC for approval. It includes specific slides for introducing the project, stating objectives and hypotheses, detailing animal requirements, explaining the rationale for animal usage, and
0 views • 9 slides
Ensuring Ethical Approval in Research Governance
Research ethics are crucial in maintaining integrity in research projects. All researchers must obtain ethical approval, following robust processes to protect interests and ensure safety. Applications for approval should be submitted through Ethics Monitor with attention to detail and timely submiss
0 views • 19 slides
Ensuring Ethical Approval for Research: Guidelines and Procedures
Research governance, ethical frameworks, and institutional procedures play crucial roles in ensuring the integrity and quality of academic research. The ESRC Research Ethics Framework outlines key principles for conducting research ethically, emphasizing transparency, participant consent, and risk m
0 views • 22 slides
Understanding Institutional Review Boards (IRB): A Comprehensive Overview
Institutional Review Boards (IRBs) play a critical role in reviewing, approving, and monitoring human subjects research to ensure their safety and rights are protected. This overview covers the functions of IRBs, different levels of review, types of studies, criteria for approval, and how IRBs deter
0 views • 12 slides
UNR WLTP: Regulations Update for Vehicle Type Approval
This document details the transposition of GTR15 (WLTP) and GTR19 (Evap) into UN Regulations, focusing on the scope, definitions, and application for approval of vehicle categories M1, M2, N1, and N2. It outlines requirements for emissions testing, carbon dioxide, fuel consumption, electric energy c
0 views • 38 slides
Guide to Academic Proposal Approval Process
Ensure department and chair support, fill out the proposal form, provide necessary information, submit to department and committees for approval, and finally obtain Provost approval for curriculum inclusion.
1 views • 11 slides
Research Ethics Approval Process at uOttawa
Manage your research ethics approval at uOttawa effectively through the Research Ethics Board (REB) process. Get guidance on submitting applications via eReviews, completing the REB evaluation checklist, and ensuring ethical standards in projects involving human participants. Explore resources for s
0 views • 11 slides
Understanding Research Ethics at the University of Liverpool
Research Ethics at the University of Liverpool play a crucial role in ensuring the dignity, rights, safety, and well-being of human participants are respected and safeguarded. Ethical approval is mandatory for all research involving human participants, animals, their tissues, or data. Consent, priva
0 views • 14 slides
Comprehensive Guide to CTE Program Approval Process
A detailed guide covering the CTE program approval process, deadlines, components of an approved CTE program of study, CIP code and program name significance, data approval applications, and self-study form requirements. It explains the application deadlines, consequences of missing documentation, l
0 views • 16 slides
Guidelines for Outside Professional Work Approval
Guidelines for seeking approval for outside professional work at the University, detailing the process for professional and classified staff as well as faculty, librarians, and other academic personnel. The procedures involve completing specific forms, obtaining supervisor and departmental approvals
1 views • 7 slides
USPS Mail Analytics Access and Approval Process
Gain access to USPS Mail Analytics by following a specific approval process involving BSA approval, request submission, and verification steps. This process ensures compliance with sensitive information handling protocols and attorney-client privilege.
0 views • 8 slides
Update on CAR T-Cell Therapy and Approval Criteria Changes
Recent updates regarding CAR T-cell therapy include details on patient referrals, treatments, and changes to approval criteria. The information highlights patient outcomes, referral processes, and criteria updates for various types of lymphomas. It also discusses the importance of monitoring and adj
2 views • 9 slides
Guidelines for Submitting New Technical Program Proposals
This presentation by Charmine Chambers, Director of Workforce Development, provides essential information on submitting new technical program proposals for approval by TEA and KBOR. It covers program approval processes, funding eligibility, program reviews, required documents, submission deadlines,
0 views • 19 slides
Safety Regulation of PBN Operations Workshop Insights
Safety regulation of performance-based navigation (PBN) operations is crucial for Instrument Rated (IR) pilots to ensure safe and efficient IFR flying. This includes specific requirements and privileges, such as minimum decision heights, airworthiness approval, mature systems, and suitable training
0 views • 24 slides
Water Quality Assessment Process Summary for EMC Approval
The request seeks EMC approval for the 2014 303(d) listing methodology by Kathy Stecker. The process involves conducting reviews, public comments, and submitting lists for EPA approval. It includes assessment categories, response to comments, statistical confidence, and criteria exceedance. Changes
0 views • 11 slides
Invoice Approval and Notification Process Quick Reference Guide
This reference guide outlines the invoice approval and notification process, detailing email alerts for approval and exceptions, with instructions on how to validate and approve invoices in Guided Buying. It also covers notifications for invoices requiring accounting information and provides guidanc
0 views • 13 slides
Enhancing Standardization with Ghana's Type Approval Management System
Exploring the success story of Ghana's Standardization Type Approval Management System at the 3rd SG13 Regional Workshop for Africa. Isaac Boateng from the National Communications Authority shared insights on the technical and regulatory requirements, challenges, and the importance of Type Approval
0 views • 18 slides
Guide to Creating a Pre-Approval Report in Chrome River
Learn how to efficiently create a pre-approval report in Chrome River for a business trip, including adding expenses such as airfare and mileage, reviewing entries, and completing the necessary forms for approval. Detailed step-by-step instructions provided with accompanying visuals.
0 views • 28 slides
Understanding the Effects of Contract on Goods Approval and Property Rights
Explore the effects of contract on goods approval, property rights, and risk transfer between buyers and sellers. Learn about passing of property, reservation of right of disposal, and risk associated with goods until transfer of ownership. Understand the significance of buyer's approval and accepta
0 views • 4 slides
Coastal Commission Meeting June 9, 2022 - Project Plans and Approval Conditions
The Coastal Commission Meeting on June 9, 2022, at the Greer Residence in Newport Beach, California, discussed the proposed development involving the removal of unpermitted structures and encroachment into the Seminuk Slough wetland. Project plans, including the demolition of a deck, were presented
0 views • 5 slides
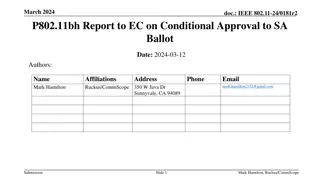
March 2024 IEEE 802.11bh Conditional Approval Report
This document presents the report to the IEEE 802 Executive Committee requesting conditional approval to send P802.11bh to SA Ballot. It includes details on the completion of comment collections and multiple working group letter ballots. The results show the achievement of over 75% approval for Draf
0 views • 10 slides
Professional Development Information & Approval Process Guidelines
Guidelines for professional development approval process from January to June 2015 include maximizing staff presence, prioritizing PD activities, determining criteria for mandatory PDs, and steps to follow for approval. The process involves rescheduling events, using specific criteria, and submittin
0 views • 9 slides
Understanding Saint Leo IRB Process
Discover the Saint Leo IRB review process, its purpose, requirements for research approval, definitions of human subjects and research, and how to apply for IRB approval. Learn about the exclusion criteria for class activities and access useful resources and guidance for conducting research at Saint
0 views • 17 slides
ASU EHS & FSE Chemical Approval Process Guidelines
Detailed guidelines and forms for the chemical approval process at ASU, including links to important resources, forms for new chemical purchases and transfers, responsibilities for lab managers, and procedures for chemical transfers. The process involves completing forms such as the Prior Approval A
0 views • 5 slides
Approval Frameworks and Forms for Academic Programs by Tamara Ferry, Ph.D.
A comprehensive guide on approval frameworks and forms for academic programs, addressing the fragmented and isolated historical practices. It includes four essential frameworks for proposing new programs, making changes to existing programs, creating professional development proposals, and discontin
0 views • 18 slides
USG Strategic Hire Approval Process effective December 15, 2019
The USG Strategic Hire Approval Process, implemented as of December 15, 2019, outlines key provisions for approving vacant and new faculty and staff positions above $40,000 salary. The process requires a critical hire justification narrative and approval from key personnel like President Keel and Yv
0 views • 14 slides
DARPA Human Subject Research Approval Process Overview
DARPA requires all research involving human subjects to undergo a rigorous approval process. This includes submission to local Institutional Review Boards (IRBs), review by DoD headquarters, and obtaining consent documents. Delays in approval can occur, especially for studies involving protected pop
0 views • 8 slides
Understanding the Animal Rule in Drug Approval Process
The Animal Rule addresses the approval of new drugs when human efficacy studies are not possible. It serves as a surrogate for human studies, requiring well-controlled animal testing to predict clinical benefits in humans. Safety must still be demonstrated in human trials, and the rule can be bypass
0 views • 27 slides
Request Approval for SBAM Game Submission Process
In this guide, learn how to submit a Skill-Based Amusement Machine (SBAM) game for approval by the Commission via the Skill Games Reporting (SGR) System. Follow step-by-step instructions on logging in, providing detailed game information, and uploading necessary documentation to support your request
0 views • 11 slides
Exploring Research Design and Funding Priorities in Northern Ireland
Dive into the world of research at the upcoming Application and Research Design Workshop scheduled for Friday, 28th May 2021. Discover the strategic priorities driving impactful research initiatives, learn about current research projects, funding processes, and collaborations. Explore the rich histo
0 views • 37 slides
Online Procedures for Travel Approval and Expense Reimbursement
Valentin Valdez provides detailed instructions on the before, during, and after processes for travel approval and expense reimbursement. From getting prior approval to creating requisitions, coding expenses, and submitting for approval, this guide covers all steps necessary for successful travel arr
0 views • 18 slides
Clinical Research Workflow Optimization Overview
Clinical research workflow optimization aims to streamline the process of subject enrollment, study approval, and Epic integration. It involves linking encounters to research studies, using automated tools for transactional data, and enhancing the research workqueue training. The process includes au
0 views • 21 slides
Ensuring Data Trustworthiness at Odum Institute
The Odum Institute showcases its trustworthiness through its DataVerse platform and Data Seal of Approval, emphasizing accessibility, reliability, and responsibility in managing research data. Researchers and archivists collaborate to ingest and curate data, ensuring usability and citability. Odum's
0 views • 17 slides