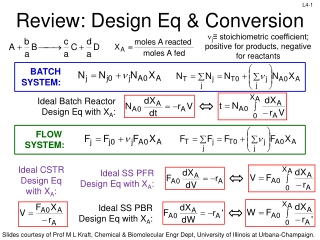
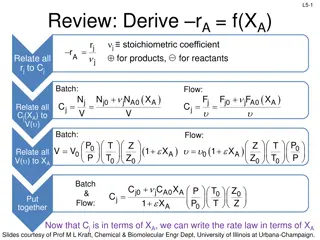
Review: Design Eq & Conversion
Design equations for batch, flow, CSTR, PFR, and PBR systems are discussed, emphasizing the calculation of conversion and reactor volumes. The importance of understanding reaction rates and stoichiometry in determining reactor sizes is highlighted, along with numerical evaluation techniques for inte
1 views • 25 slides
Stoichiometry in Chemistry
Stoichiometry in chemistry involves calculating quantities of reactants and products in a chemical reaction based on a balanced equation. This process ensures the conservation of mass and atoms. Mole ratios are used as conversion factors, and steps such as converting to moles and applying the mole r
1 views • 25 slides
Stoichiometry Test Review Sheet
A comprehensive review sheet covering key concepts in stoichiometry, including molar ratios, coefficients in chemical equations, determining actual yield in reactions, identifying limiting reactants, calculating percentage yield, and understanding mole ratios in chemical reactions. The review provid
0 views • 11 slides
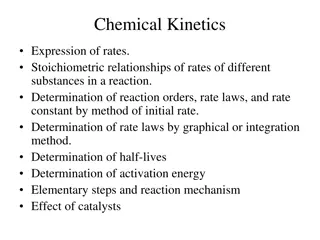
Chemical Kinetics: Rates, Reactions, and Mechanisms
Chemical kinetics involves studying reaction rates, rate laws, stoichiometry, and factors affecting reaction speed. This branch of chemistry delves into determining reaction orders, rate constants, and activation energies using various methods. Different types of rates, such as initial, instantaneou
5 views • 68 slides
Acid-Base Titration in Chemistry
Acid-base titration is a quantitative technique used to determine the concentration of an unknown acid or base solution. By neutralizing the solution with the opposite component and reaching the equivalence point, students can calculate the concentration using stoichiometry principles. This method d
1 views • 24 slides
Acid-Base Titration in Chemistry
Acid-base titration is a quantitative technique used to determine the concentration of unknown acids or bases. Through neutralization reactions, the concentration of a solution can be calculated based on the stoichiometry of the reaction. This method dates back to the late 18th century and is crucia
0 views • 24 slides
Comprehensive Alignment of MOE Chemistry Syllabus with Khan Academy Resources
This comprehensive alignment showcases the correlation between the Ministry of Education (MOE) chemistry syllabus and the educational resources provided by Khan Academy. It covers various topics in chemistry such as the particulate nature of matter, atomic structure, chemical bonding, stoichiometry,
1 views • 9 slides
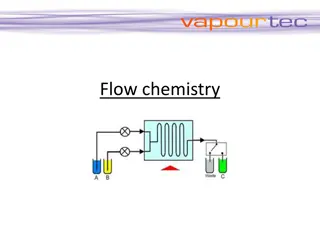
Flow Chemistry for Efficient Chemical Reactions
Flow chemistry, also known as continuous flow or plug flow chemistry, revolutionizes chemical reactions by running them in a continuous flow stream. This dynamic process offers efficient manufacturing of chemical products with precise control over critical parameters like stoichiometry, mixing, temp
2 views • 7 slides
Stoichiometry in Chemical Reactions
Stoichiometry is the concept of predicting the amounts of reactants and products in a chemical reaction, similar to following a recipe in cooking. It involves balancing chemical equations and determining the quantities of substances involved. By paying attention to coefficients, one can calculate ho
3 views • 65 slides
Fundamentals of Chemical Kinetics and Reactor Design
Explore the realm of chemical reactions, rate equations, and reactor design in this informative chapter. Understand the factors influencing reaction rates, different types of reactions, rate laws, and experimental determination of reaction rates. Dive into examples illustrating stoichiometry and rat
0 views • 19 slides
Chemical Reactor Design Principles
Explore the fundamentals of chemical reactor design, including stoichiometry, reaction rates, and reactor scale-up. Learn to derive rate laws and design equations in terms of conversion for batch, CSTR, and PFR reactors. Discover the logic behind isothermal reactor design and calculation of required
0 views • 21 slides
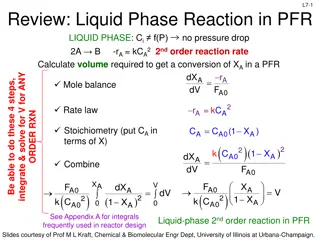
Reactor Design Principles for Liquid and Gas-Phase Reactions
This material covers the design principles for liquid and gas-phase reactions in continuous flow reactors such as PFR (Plug Flow Reactor) and PBR (Packed Bed Reactor). It includes calculations for volume required to achieve a specific conversion, catalyst weight needed, and considerations for ideal
0 views • 21 slides
Calculating Limiting Reagent in Chemical Reactions
Calculating the amount of reactants in excess and the limiting reagent plays a crucial role in determining the maximum extent of a chemical reaction. By using the relative numbers of moles of substances as shown in balanced equations, one can identify the reactant that is fully utilized, hence limit
0 views • 14 slides
Insights on Biogeochemical Processes and Metal Interactions in Marine Environments
Delve into the intricate relationships between metals, stoichiometry, and biological quotas in marine ecosystems. Explore the impact of metal interactions on biogeochemical provinces, resource supply stoichiometry, and the constraints they impose on marine biota. Uncover how deep chlorophyll maxima,
0 views • 5 slides
Nitrogen Dynamics in a Mediterranean Savanna Ecosystem
Investigating the fate of nitrogen from fertilizer treatments and root litter turnover in a Mediterranean Savanna ecosystem. The study compares the short-term fate of 15N tracers in ecosystem stoichiometry experiments, highlighting changes in soil-plant functioning. The research addresses N turnover
0 views • 10 slides
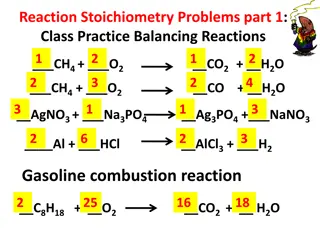
Stoichiometry in Reactions: Balancing Equations and Real-World Applications
Stoichiometry in reactions involves balancing equations, understanding coefficients, and controlling fuel injectors in the real world. This concept is crucial for chemistry, fuel combustion, and cooking, as shown through various examples and problems provided.
0 views • 6 slides
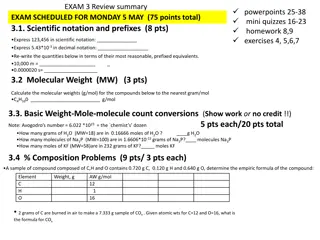
Chemistry Exam Review: Topics in Scientific Notation, Molecular Weight, Stoichiometry, and Limiting Yield
Explore key concepts in chemistry, including scientific notation, molecular weight calculations, reaction balancing, stoichiometry, and limiting yield problems. Prepare for an upcoming exam by practicing various problems and conversions related to these topics, such as expressing numbers in scientif
1 views • 4 slides
Stoichiometry in Chemical Reactions
Stoichiometry in chemical reactions involves mass changes, limiting reactants, and calculating yields like moles and grams. Learn to solve problems involving different reactants and products, determining the quantities involved in a reaction. Examples cover decompositions, formations, and calculatio
1 views • 22 slides
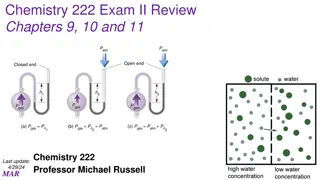
Chemistry 222 Exam II Review: Gas Laws, Reactions, and Intermolecular Forces
Explore questions related to gas volume changes with pressure, gas density comparisons, stoichiometry in gas reactions, gas velocity comparisons, pressure comparison between gases, boiling point predictions based on intermolecular forces, and ranking molecules based on intermolecular forces.
0 views • 28 slides
Introduction to Chemical Reaction Engineering (CRE)
Chemical Reaction Engineering (CRE) focuses on studying the rates and mechanisms of chemical reactions, as well as designing reactors for these reactions. The field involves understanding balances in terms of molar flow rates, mole balances, rate laws, stoichiometry, and membrane reactors. Membrane
0 views • 20 slides
Comprehensive Overview of Ideal Gas Law and Gas Problems
Delve into a detailed exploration of the Ideal Gas Law and its applications in solving various gas-related problems. Master the concepts of Boyle's Law, Charles's Law, Avogadro's Law, and more through equation, ratio, and stoichiometry problems. Enhance your understanding of gas behavior and calcula
0 views • 20 slides
Stoichiometry Performance Assessment: How Much Baking Soda Do You Need?
The experiment aims to determine the amount of baking soda required to produce a target quantity of sodium carbonate through a thermal decomposition reaction. Students will balance the chemical equation, calculate the necessary NaHCO3 amount, heat the mixture, and compare theoretical versus actual y
1 views • 14 slides
Chemical Reactions and Stoichiometry in AP Chemistry
Chemical reactions involving stoichiometry play a crucial role in AP Chemistry. Understanding how atoms are rearranged, mass is conserved, and energy is exchanged is essential for balancing chemical equations. Examples include the thermite reaction, combustion of methane, and reactions like synthesi
0 views • 38 slides
Stoichiometry Calculations in Chemistry
Explore various stoichiometry calculations including determining the number of moles in given volumes and masses, calculating expected product masses based on balanced equations, and finding the mass of products produced in chemical reactions. Practice examples with magnesium, carbon dioxide, methan
2 views • 13 slides
R.I.C.E. Tables and Stoichiometry for Limiting Reactants
R.I.C.E. tables play a crucial role in chemistry, particularly in stoichiometry when dealing with reactions and limiting reactants. This method involves organizing information and setting up equations to find unknowns. An example is provided with the combustion of ethene to determine the volume of c
0 views • 35 slides
Stoichiometry and The Mole Concept in Chemistry
Explore the fundamentals of stoichiometry and the mole concept in chemistry, including conversions between moles and particles, molar mass calculations, and gram mole conversions. Learn how to determine the number and kinds of atoms in chemical formulas and understand the significance of Avogadro's
1 views • 12 slides
Nutrient Conceptual Model Review Summary
Comments and feedback on a Nutrient Conceptual Model report include suggestions to address issues related to eutrophication concepts, nutrient stoichiometry, community composition, and nutrient reduction goals. Recommendations also focus on incorporating flushing/residence time discussions, predicti
1 views • 9 slides
Introduction to Chemical Reaction Engineering
Chemical Reaction Engineering (CRE) is crucial for understanding how chemical reactors operate in various processing operations. This field involves reactor design by integrating factors such as thermodynamics, kinetics, fluid mechanics, heat transfer, and economics. CRE aims to effectively design a
0 views • 16 slides
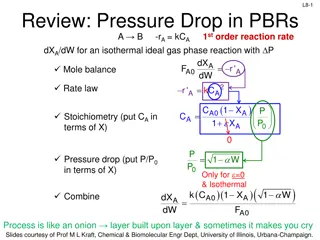
Reactor Design Fundamentals: Rate Laws and Analysis
This content covers the analysis of pressure drop in packed bed reactors, determining reaction order, and studying rate data for reactor design. It delves into stoichiometry, kinetics, fluid dynamics, and the challenges of collecting and analyzing rate data. Examples of rate laws and reactions are p
2 views • 32 slides
Chemistry Study Materials and Practice Questions
This collection of chemistry study materials includes information on reactions, solubility, electrolytes, and solution stoichiometry. Practice questions cover classifying solutes, predicting solubility of salts, writing ions produced in water, and determining precipitates in reactions. Includes a mo
0 views • 15 slides
Mastering Stoichiometry: Definitions, Calculations, and Limiting Reactant
Dive into the world of stoichiometry with essential concepts like limiting reactants, theoretical yield, and percent yield. Practice calculations and sharpen your skills through real-world examples like making pizza and chemical reactions. Explore the intricacies of balancing equations and determini
1 views • 20 slides
Chemistry Lecture Announcements and Stoichiometry Concepts
In this lecture, the announcements cover important class updates and quizzes, while delving into the fundamental concept of stoichiometry in chemistry. Stoichiometry involves understanding the ratios of reactants and products in chemical reactions. The text explores error and uncertainty in measurem
0 views • 18 slides
Stoichiometry in Chemistry
The study of mathematical relationships in balanced chemical reactions for calculating reactant and product amounts, applying skills to convert between moles and masses of different compounds. Explore coefficients, mole ratios, and examples to understand stoichiometry fundamentals.
0 views • 4 slides
STOICHIOMETRY BOARD RELAY
In this stoichiometry board relay activity, players set up problems, work through conversion factors, predict reaction products, calculate moles of products formed, and determine the mass of resulting compounds. Using reactions involving sodium carbonate, barium nitrate, barium carbonate, and sodium
0 views • 32 slides
Stoichiometry - The Mathematics of Chemical Reactions
Stoichiometry is a crucial aspect of chemistry, involving the quantitative relationships in chemical reactions. This concept delves into the calculations of reactants and products based on balanced chemical equations, helping to determine the amounts of substances involved. By understanding stoichio
0 views • 36 slides
Stoichiometry in Chemical Reactions
Stoichiometry explores the proportions in which chemical species combine, guiding the calculation of reactants and products in reactions. Key terms include stoichiometric equation, coefficient, ratio, proportion, limiting reactant, excess reactant, and percent excess. Understanding these concepts is
0 views • 14 slides
Chemical Equations and Stoichiometry Concepts
Explore the fundamentals of stoichiometry and chemical equations in this comprehensive guide. Learn about the arithmetic of equations, conservation laws, mole ratios, and solve practice problems. Enhance your understanding of chemical calculations with detailed explanations and examples.
0 views • 33 slides
Understanding Stoichiometry for Chemical Reactions
Learn about stoichiometry, the process of converting known quantities of substances into unknown quantities using balanced equations. Discover the importance of mole ratios and how to apply them in stoichiometric calculations. Practice solving stoichiometry problems with real-world examples.
0 views • 14 slides
Understanding Solution Stoichiometry in Chemistry
Explore the concepts of solution stoichiometry in chemistry, including molarity calculations, interconverting moles and volume, solution stoichiometry practice questions, and more. Learn how to determine concentrations of aqueous solutions and conduct titrations for acid-base reactions.
1 views • 13 slides
Understanding Stoichiometry in Chemistry
Explore the concept of stoichiometry through examples involving chemical compounds such as LaO0.5F0.5BiS2, CeRhIn5, and CaCu3Ru4O12. Understand how to balance chemical equations, calculate molar masses, and normalize compounds for 1g. Dive into the world of chemistry with this detailed analysis.
0 views • 7 slides