Stoichiometry Calculations in Chemistry
Explore various stoichiometry calculations including determining the number of moles in given volumes and masses, calculating expected product masses based on balanced equations, and finding the mass of products produced in chemical reactions. Practice examples with magnesium, carbon dioxide, methane, and carbon compounds.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
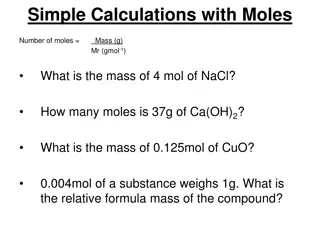
Starter Calculate the number of moles: 1. In 100 cm3of concentration 0.1 mol l-1 2. Of 12g of magnesium sulfate (MgSO4) 3. Of 20g of carbon dioxide (CO2) 4. In 200 cm3of concentration 2 mol l-1
Learning Intention Learn how the theoretical mass of product can be calculated from the balanced reaction equation. By the end of today you should be able to: Calculate the mass of a product based on a balanced equation
From previous studies You should be able to Write Formulae Calculate percentage composition Calculate empirical formulae Calculate the number of moles in a given mass Calculate the number of moles of solute dissolved in a solution
Reacting Masses Accurately weigh a crucible Add approx 1.2g of Mg ribbon and reweigh Place the crucible and lid in a silica triangle. Heat gently at first then more strongly. Lift the lid with tongs from time to time to admit more oxygen, but not enough to let out the magnesium oxide.
Mass (g) Mass of crucible Mass of crucible + Mg ribbon Calculated mass of Mg ribbon Mass of crucible + MgO Calculated mass of MgO
Reacting masses When the reaction is complete, the magnesium will not glow more brightly when the lid is raised Allow the crucible to cool Reweigh the crucible
Calculations Using the balanced equation calculate the mass of MgO you would expect to be formed? 2Mg + O2 2MgO
Reacting masses Calculate the mass of carbon dioxide produced when 64g of methane is burned according to the following equation. CH4 + 2O2 CO2 + 2H2O 176g
Reacting masses Calculate the mass of carbon dioxide produced when 48g of carbon is burned according to the following equation. C + O2 CO2 176g
Reacting masses What mass of zinc sulphate will be produced on adding 6.0g zinc to excess sulphuric acid? 14.9g
Reacting masses What mass of sodium carbonate will react completely with 100cm3 of nitric acid concentration 1 mol l-1? 5.3g
You can calculate the number of moles (n) in a substances How many moles in 18g of carbon? 1 mole Carbon (18/12) x 1 = 1.5 moles 12g 18g n = mass/GFM n= 18/12 n= 1.5 moles
Solutions 1. Calculate the mass of KOH required to make up 0.5 litres of a solution with a concentration of 2 mol l-1. 2. Calculate the mass of Na2CO3 required to make a 500 cm3 solution of concentration 3 mol l-1. 3. Calculate the concentration of a solution where 12.8g of SO2 is dissolved in 2 litres of solution. 4. Calculate the concentration of a solution where 11.2 of KOH is dissolved in 100 cm3 of solution 56.1g 159g 0.1 mol l-1 2 mol l-1