Reactor Design Principles for Liquid and Gas-Phase Reactions
This material covers the design principles for liquid and gas-phase reactions in continuous flow reactors such as PFR (Plug Flow Reactor) and PBR (Packed Bed Reactor). It includes calculations for volume required to achieve a specific conversion, catalyst weight needed, and considerations for ideal gas-phase reactions in tubular reactors. The content discusses mole balance, rate laws, stoichiometry, and key concepts related to second-order reactions.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
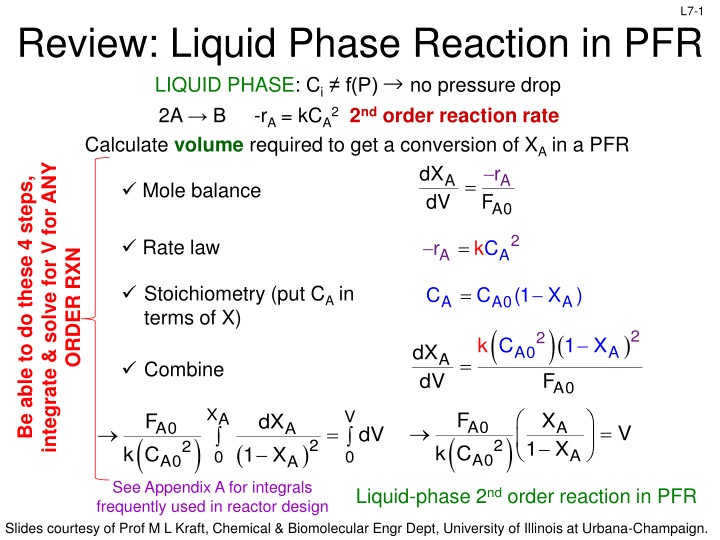
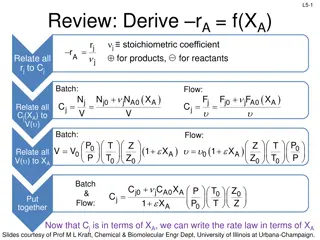
L7-1 Review: Liquid Phase Reaction in PFR LIQUID PHASE: Ci f(P) no pressure drop 2A B -rA = kCA22nd order reaction rate Calculate volume required to get a conversion of XA in a PFR integrate & solve for V for ANY F d X dV r Be able to do these 4 steps, A A = Mole balance A0 2 = Rate law r kC A A ORDER RXN Stoichiometry (put CA in terms of X) = C C (1 X ) A A0 ( A )( F 2 ) 2 k C 1 X d X d A0 A A = Combine V A0 X V F X F dX A A0 A A0 A = = V dV ( ) ( ) 2 2 1 X 2 ( ) k C k C 1 X A 0 0 A0 A0 A See Appendix A for integrals frequently used in reactor design Liquid-phase 2nd order reaction in PFR Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
L7-2 Review: Liquid Phase Reaction in PBR LIQUID PHASE: Ci f(P) no pressure drop 2A B -r A = kCA22nd order reaction rate Calculate catalyst weight required to get a conversion of XA in a PBR Be able to do these 4 steps, integrate F d dW X r ' & solve for V for ANY ORDER RXN A A = Mole balance A0 2 = Rate law r' kC A A Stoichiometry (put CA in terms of X) = C C (1 X ) A A0 ( A )( F 2 ) 2 k C 1 X d X W d A0 A A = Combine A0 X F X W F dX A A0 A A0 = A W = dW ( ) 0 0 ( ) 2 1 X 2 2 ( ) k C k C 1 X A A0 A0 A Liquid-phase 2nd order reaction in PBR Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
Review: Isobaric, Isothermal, Ideal Gas-Phase Rxns in Tubular Reactors L7-3 Gas-phase reactions are usually carried out in tubular reactors (PFRs & PBRs) Plug flow: no radial variations in concentration, temperature, & -rA No stirring element, so flow must be turbulent FA0 FA + + + + C C X C C X 1 1 T T Z Z P P j0 A0 X j A j0 A0 X j A 0 0 = = C C GAS PHASE: j j 1 1 A A 0 1 Stoichiometry for basis species A: ( ) + C 1 X X C C X A0 1 A A0 A0 X A = = C C A A + 1 A A Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
L7-4 Review: Effect of on and XA Tf T0 N N Change in total # moles at X N = 1 A = = total moles fed T0 : expansion factor, the fraction of change in V per mol A reacted 0: volumetric flow rate varies if gas phase & moles product moles reactant, or if a P, T, or Z occurs No P, T, or Z occurs, but moles product moles reactant = 0 (mol product = mol reactants): = : constant volumetric flow rate as XA < 0 (mol product < mol reactants): volumetric flow rate as XA Q1: For an irreversible gas-phase reaction, how does the residence time and XA change when < 0? a)They don t b)The residence time is longer & XA increases c)The residence time is longer & XA decreases d)The residence time is shorter, & XA decreases e)The residence time is shorter & XA increases ( 0 P P 1 Z Z T T = ( ) 0 = + 1 X 0 A 0 0 ) + X A Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
L7-5 Review: Effect of on and XA Tf T0 N N Change in total # moles at X N = 1 A = = total moles fed T0 : expansion factor, the fraction of change in V per mol A reacted 0: volumetric flow rate varies if gas phase & moles product moles reactant, or if a P, T, or Z occurs No P, T, or Z occurs, but moles product moles reactant = = 0 (mol product = mol reactants): = : constant volumetric flow rate as XA increases < 0 (mol product < mol reactants): volumetric flow rate decreases as XA increases Longer residence time than when = Higher conversion per volume of reactor (weight of catalyst) than if = 0 > 0 (mol product > mol reactants): with increasing XA Shorter residence time than when = Lower conversion per volume of reactor (weight of catalyst) than if = 0 P P Z Z T T ( ) 0 = + 1 X 0 A 0 0 ( ) + 1 X 0 A Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
Review: Isobaric, Isothermal, Ideal Rxn in PFR GAS PHASE: Ci = f( ) no P, T, or Z Calculate PFR volume required to get a conversion of XA Be able to do these 5 steps, & solve for V L7-6 2A B -rA = kCA22nd order reaction rate F d X dV r A A = Mole balance A0 2 = Rate law r kC A A 1 X X for ANY ORDER RXN ( ) C A0 1 A Stoichiometry (put CA in terms of X) = C A + A 2 2 ( ) ( X ) 2 k C 1 X dX d A0 A A = Combine ( ) V + 1 F A A0 2 ( ) 2 XA + 1 X F A A0 = V dX Integral A-7 in appendix ( ) A 2 ( ) 1 X k C 0 A0 A Gas-phase 2nd order rxn in PFR no P, T, or Z )2 ( + 1 X F ( ) ( ln 1 X ) 2 A A0 = + + + V 2 1 X ( ) A A 1 X 2 k C A A0 Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
L7-7 Review: Pressure Drop in PBRs GAS PHASE: A B -r A = kCA2 Calculate dXA/dW for an isothermal ideal gas phase reaction with P 2nd order reaction rate dX d = A = F r' Mole balance A0 A W kC 2 Rate law r' A A ( ) X C 1 X ) ( ( 1 P P A0 1 A = C Stoichiometry (put CA in terms of X) A + A 0 ( 2 2 2 k C ) 1 X dX dW P P A0 A Combine A = 2 ) F + X A 0 0 A P dP dW T Relate P/P0 to W (Ergun equation) )( ) 0 = + 1 X A ( 2 T dy dW P P 0 0 Ergun Equation can be simplified by using y=P/P0 and T=T0: Simultaneously solve dXA/dW and dP/dW (or dy/dW) using Polymath ( ) = + 1 X A 2y Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
L7-8 Review: Ergun Equation P dP dW T Calculates pressure drop in a packed bed. This equation can be simplified to: )( ) 0 = + 1 X A ( 2 T P P 0 0 dy dW T T Differential form of Ergun equation for pressure drop in PBR: ( ) = + 1 X A 2y 0 N N 2 P P Tf T0 0 = = = = y y A0 ( ) c c N A 1 P 0 T0 0 volume of solid ( ) 1 : fraction of solid in bed =total bed volume C: particle density AC: cross-sectional area : constant for each reactor, calculated using a complex equation that depends on properties of bed (gas density, particle size, gas viscosity, void volume in bed, etc) : constant dependant on the packing in the bed Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
L7-9 L7: Unsteady-State Isothermal Reactor Operation: CSTR Start-Up and Semi-Batch Reactors CB 0 Semi-batch A A+B V0 + 0t V0 Vf end time t start Time required to reach steady-state after CSTR start-up Predicting concentration and conversion as a function of time Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
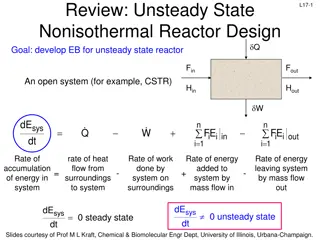
L7-10 Start-Up of a Fixed-Volume CSTR Isothermal (unusual, but simple case), well-mixed CSTR Unsteady state: concentrations vary with time & accumulation is non-zero Goal: Determine the time necessary to reach steady-state operation 0CA CA0 0 In Out - +Generation = Accumulation moles A in CSTR changes with time until steady state is reached dN dt A + = F F r V A0 A A Use concentration rather than conversion in the balance eqs + C C r V dN d 1 V dN dt A0 0 A 0 A A t = A A0 0 A 0 + = C C r V A V Divide by V to convert dNA to dCA V C C d C dt dC dt Multiply by A0 A A = A + = r + = C C r A A0 A A 0 Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
L7-11 CSTR Start-Up: 1st Order Reaction + = A A0 A C C dt A Integrate this eq to find CA (t) while 1st order rxn in CSTR is at unsteady-state: dC dC dt A A Combine r = C C k C = Ar kC A0 A A Bring variables to one side & factor dC dt C 1 k + 1C ( ) ( ) A = 1 k + C A0 A dC dt 1 Put like variables with their integrals ( )( A0 A = 1 k + C A ) C 1 k C 1 k A0 + ( ) C C t + 1 k dC A A ( ) ( ) A + 1 k = dt = ln 0 t C 1 k A0 + 0 0 C A0 + A ( ) 0 ( ) ( ) + 1 k t C C 1 k A A0 + ) = ( 1 e C 1 k ( ) + t 1 k A0 + 1 e = C A ( ) Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
L7-12 CSTR Start-Up: 1st Order Reaction dC C C kC dt CSTR of 1st order rxn is in unsteady-state: At steady state, t is large and: 0 We integrated this eq to find CA (t) while A + = A0 A A ) ( C 1 k C 1 k ( ) + t 1 k A0 A0 + = 1 e = C C AS A + dC dt Is this consistent with steady state balance eq for CSTR? No accumulation at steady state A = C C kC A0 A A Yes, same! 0 C 1 k A0 + C 1 k + Goal: combine start-up and SS eqs to estimate time to reach SS (ts) = = C C kC 0 C A0 A A AS ( ) ) ( C 1 k + In the unsteady state, when CA = 0.99CAS: Solve for ts to determine time to reach 99% of steady-state concentration ( ) 1 k + t A0 A 0 s 1 e = 0.99 ) ( ( ) ( ) ( ) 1 k + 1 k + 1 k + t t t ( ) s s s = = = 1 e 0.99 0.01 e ln 0.01 ln e + 1 k time to reach 99% of steady-state concentration in terms of k = = 4.6 t 4.6 t s s 1 k + Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
L7-13 CSTR Start-Up: 1st Order Reaction ( 1 k + ) C ( ) + t 1 k A0 1 e = C A In the unsteady state, the time to reach CA = 0.99CAS is: = t 4.61 +k When k is very small (slow rxn), 1>>k : When k is very big (fast rxn), 1<<k 4.6 k = 4.6 = st t s + 63% of the steady-state concentration is achieved at: 1 k CA = 0.63CAS 99% of the steady-state concentration is achieved at: = 4.6 C 0.99C A AS 1 k + Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
Better Selectivity in a Semi-Batch Reactor To enhance selectivity of desired product over side product L7-14 2 kP = P r k C C Desired product P + A B P p A B 2 kS = S r k C C Undesired side product S + A B S S A B Instantaneous selectivity, SP/S, is the ratio of the relative rates*: 2 P P A P/S S S A k C C r r k C C k C B 2 P A = = = S S B k C B Higher concentrations of A favor formation of the desired product P Higher concentrations of B favor formation of the undesired side product S To maximize the formation of the desired product: Slowly feed B into the reactor containing A Commonly used in bioreactors, when the enzyme is inhibited by excess substrate *We ll look at this concept of instantaneous selectivity in more detail in L9 Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
L7-15 Semi-Batch Reactor Design Equation Do a mole balance on A since it does not enter or leave the reactor (assume the reactor is well-mixed) In Out - + Generation CB 0 Accumulation dN dt d dt = A + = F F r V A0 A A N A + = 0 0 r V A V0 + 0t Use whatever units are most convenient (NA, CA, XA, etc) Convert NA to CA using: d C dt V N A = r V A = = C N C V A A A A V 2 parts: how CA changes with t and how V changes with t dC d dV dt A t = + r V V C A A Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
L7-16 Semi-Batch Reactor Design Equation Do a mole balance on A since it does not enter or leave the reactor (assume the reactor is well-mixed) - + Generation CB 0 In Out Accumulation = dN dt ( ) A + = 0 0 r V t A dC dt dV dt 2 parts: how CA changes with t and how V changes with t A = + r V V C A A V0 + 0t Reactor volume at any time can be found with a mole balance - In Out + Generation Accumulation ( ) V dt = d = 0 = dV dt = 0 0 + = 0 0 + = V t V 0 0 0 dC dt A Substitute: = + r V V C Rearrange to get in terms of dCA/dt A A 0 dC dt C dC dt A A 0 V A A 0 = r V C V = r Balance on A A A Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
L7-17 Semi-Batch Reactor Design Equation Mole Balance on B In Out - + Generation CB 0 Accumulation dN dt = B + = B0 F 0 r V B dN dt dN dt B B = + = + B r V B0 F B r V B0 F dVC dt d d ( ) B V0 + 0t = = + C V B r V B0 F B t dV dt dC d dV d = B t + = + C V B r V B0 0 C Substitute 0 B t dC dt B + = + B 0 C V B r V B0 0 C Rearrange to get in terms of dCB/dt ( ) C C dC dt 0 B0 V B B Balance on B = + B r Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
L7-18 Semi-Batch Reactor Design Equation: in Terms of NA In Out - + Generation CB 0 Accumulation = dN dt A + = 0 0 r V A dN dt = + Substitute V V t A = r V 0 0 A Reactor design eq. provided that rA is a function of NA V V dN dt ( ) A = + r V t A 0 0 V0 + 0t N N A B N N V A B = = r k r k -rA = kACACB A A 2 ( ) + t 0 0 dN d A B N kV N dN dt A t = A =A r V + t 0 0 dN dt A B N N V + dN dt B B = + k B0 F = + NB comes from BMB: r V B0 F A t 0 0 The design eq in terms of XA can be messy. Sometimes it gives a single equation when using Nj or Cj gives multiple reactor design equations. Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
Improving Yields of Reversible Rxns with Semi-Batch Reactors L7-19 FD Semi-batch A+B A+B C+D V0 - 0t V0 Vf ( ) To improve product yield in a reversible reaction: ( ) Start with A(l) and B(l) in the reactor D(g) bubbles out of the liquid phase, pushing the equilibrium to the right and forcing the reaction to go to completion Common industrial reaction: ( ) ( ) + + A l B l C l D g Boil off water to produce high MW polymer + n + H2O nylon n Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
Improving Yields of Reversible Rxns with Semi-Batch Reactors L7-20 FD Semi-batch A+B A+B C+D V0 - 0t V0 Vf ( ) To improve product yield in a reversible reaction: ( ) Start with A(l) and B(l) in the reactor D(g) bubbles out of the liquid phase, pushing the equilibrium to the right and forcing the reaction to go to completion ( ) ( ) + + A l B l C l D g How do we account for the loss of product D in the material balance? Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.
L7-21 Loss of Mass in Semi-Batch Reactor D(g) ( ) ( ) ( ) ( ) + + = 0 = A l B l C l D g elementary rxn Overall Mass balance: - + Generation =Accumulation 0 + = m V Divide mass balance by In Out g dm = m m gas leaving reactor dt 0 time m want in terms of dV/dt = dt From stoichiometry, rD = -rA Next, convert units to: time = = V m m d m 1 d dt V V0 + 0t 0 = 0 Relate to a rate: moles volume time Conversion 2: mass moles time ( ) V = = r r Conversion 1: A A mass ti me mass MW ( ) V MW m A r = = ( ) D = = moles moles MW mass D D D D D ( Substitute for ) r V MW dV dt A D One of the diff. eq. that are simultaneously solved (by Polymath) = + 0 Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois at Urbana-Champaign.