Stoichiometry Test Review Sheet
A comprehensive review sheet covering key concepts in stoichiometry, including molar ratios, coefficients in chemical equations, determining actual yield in reactions, identifying limiting reactants, calculating percentage yield, and understanding mole ratios in chemical reactions. The review provides examples and explanations to help students prepare for stoichiometry tests.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
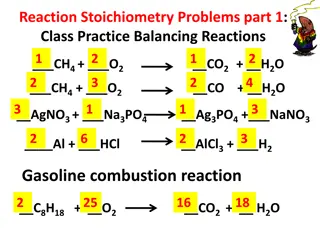
Chapter 9 Stoichiometry Test REVIEW SHEET
1. A balanced chemical equation allows one to determine the _____ ________ between all compounds in the equation. 1. MOLAR RATIOS 2. The coefficients in a chemical equation represent the ________ ____ ________ of reactants and products. 2. RELATIVE NUMBER OF MOLES 3. Actual yield of a product in a reaction must be determined by_________________________. 3. EXPERIMENTS 4. Knowing the mole ratio of a reactant and product in a chemical reaction would allow you to determine the __________ and ____________ of a product. 4. MOLES AND MASS
5. To determine the limiting reactant in a chemical reaction involving masses of the two reactants, how many products should be used in your molar ratios? 5. ONLY 1 OF THE PRODUCTS 6. How many mole ratios can be correctly obtained from the chemical equation 2Al2O3(l) 4Al(s) + 3O2(g)? 6. SIX (2:4, 2:3, 4:3, 3:4, 3:2, 4:2) 7. In the equation 2Al2O3 4Al + 3O2, what is the mole ratio of aluminum to oxygen? 7. 4:3 8. What is the name of the reactants in a chemical reaction that restricts how much product can be made? 8. LIMITING REACTANT
9. If the percentage yield for a chemical reaction is 80.0%, and the theoretical yield is 100 grams, what is the actual yield of product? 9. 80/100 X 100 = 80% 80 GRAMS 11. What is the mole ratio of H2O to H3PO4 in the following chemical equation? P4O10 + 6H2O 4H3PO4 11. 6:4 OR 3:2
12. In the formation of silicon carbide represented by the chemical equation SiO2(s) + 3C(s) SiC(s) + 2CO(g), 8 mol of each reactant are available for the reaction. What substance is the excess reactant? 12.8mol SiO2 = 1 = 8 mol SiC x 40g = 320 g SiC 1 1mol 8mol C = 1 = 2.67 mol SiC x 40g = 106.7 g SiC 3 1mol C IS THE LIMITING REACTANT SiO2 IS THE EXCESS REACTANT
13. For the reaction represented by the equation SO3 + H2O H2SO4, what is the percentage yield if 500.0 g of sulfur trioxide react with excess water to produce 575 g of sulfuric acid? Element Molar mass Hydrogen 1.01 g/mol Oxygen 16.00 g/mol Sulfur 32.07 g/mol 13. 500g SO3 x 1mol = 6.24mol SO3 = 1 = 6.24 mol H2SO4 x 98.09 80.07g 1 1mole = 612.08 grams of H2SO4: THEORETICAL YIELD 575g x 100 = 93.94% 612.08g
14. If the following reaction were to take place C5H12 + O2 CO2 + H2O a. Write the balanced chemical equation 14A. C5H12 + 8O2 5CO2 + 6H20 b. What are all of the possible mole ratios? 14B. 1:8, 1:5, 1:6, 8:5, 8:6, 5:6, 8:1, 5:1, 6:1, 5:8, 6:8, 6:5 14C. 3 mol C5H12 = 5 = 15 mol CO2 x 44g = 660 g CO2 1 c. Find the limiting reactant if 3 mol of C5H12 reacts with 2.3 mol of O2 1mol 2.3 mol O2 = 5 = 1.4375 mol CO2 x 44g = 63.25g CO2 8 1mol O2 IS THE LIMITING REACTANT
14. d. How much excess reactant would there be? 14D.2.3 mol O2 = 1 = .2875 mol C5H12 ACTUALLY USED UP 8 3 mol C5H12 - .2875 mol C5H12 = 2.7125 excess 14. e. How many grams of water would you expect to form? 14E.2.3 mol O2 = 6 = 1.725 mol H2O x 18g = 31.05g H2O 8 1mol
15. If the following reaction were to take place Al + Fe3N2 AlN + Fe a. Write the balanced chemical equation 15A. 2Al + Fe3N2 2AlN + 3Fe 15B. 2:1, 2:2, 2:3, 1:2, 1:3, 2:3, 1:2, 2:2, 3:2, 2:1, 3:1, 3:2 b. What are all of the possible mole ratios? c. Find the limiting reactant if 1.7 mol of Al reacts with .6 mol of Fe3N2 15C. 1.7mol Al = 3 = 2.55mol Fe x 54g = 137.7 g Fe 2 1mol .6mol Fe3N2 = 3 = 1.8mol Fe x 54g = 97.2 g Fe 1 1mol Fe3N2 IS THE LIMITING REACTANT
15d. How much excess reactant would there be? .6mol Fe3N2 = 2 = 1.2mol Al ACTUALLY USED UP 1 1.7 mol 1.2 mol = .5mol excess Al 15e. How many grams of iron would you expect to form? .6mol Fe3N2 = 3 = 1.8mol Fe x 54g = 97.2 g Fe 1 1mol
16. Calculate the percentage yield for the reaction represented by the equation CH4 + 2O2 2H2O + CO2 when 1000.0 g of CH4 react with excess O2 to produce 2300.0 g of CO2. 1000g CH4 x 1mol = 62.5mol CH4 = 1 = 62.5mol CO2 16g 1 62.5 mol CO2 x 44g = 2750g C : THEORETICAL YIELD 1 mol 2300g x 100 = 83.64% 2750g