Fundamentals of Chemical Kinetics and Reactor Design
Explore the realm of chemical reactions, rate equations, and reactor design in this informative chapter. Understand the factors influencing reaction rates, different types of reactions, rate laws, and experimental determination of reaction rates. Dive into examples illustrating stoichiometry and rate calculations to enhance your understanding of kinetics and reactor design principles.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Kinetics and Reactor Design CHE-402 INSTRUCTOR: Dr. INSTRUCTOR: Dr. Nabeel Salim Abo Nabeel Salim Abo- -Ghander Ghander C h a p t e r 1 Chemical Reactions and Rate of Reactions
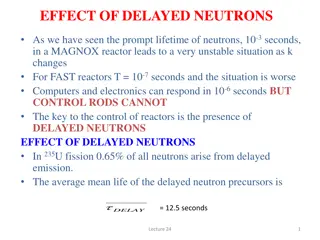
Introduction Kinetics and Reactor Design Confines reactions take place. Chemical kinetics is defined as rate of chemical reactions, that is, with the description of how fast chemical reactions occur, and factors affecting these rates. where chemical Obtain sizing parameters such as area, length, volume etc quantitative OR A device in which change in composition of matter occurs by chemical reactions 2
Basic Definitions Chemical Reaction: Classification of Chemical Reactions Phase Reversibility Molecularity 1. Unimolecular reactions: 1. Homogeneous reactions A B 1. 2. Reversible reactions Irreversible reactions 2. Heterogeneous reactions 2. Bimolecular reactions: 2A B + A B C 3. Termolecular reaction 2A B + C 3
Chemical Reactions and Rate of Reactions: aA bB + + cC dD Rate of reaction is change of concentration with respect to time! The rate equation (i.e. rate law) is an algebraic equation that is solely a function of the properties of the reacting materials and reaction conditions including species, temperature, pressure or type of catalyst, if any at a point in the system. Reaction rate definition is independent of reactor type. Mathematically, it can be represented as: ( ) ( k T f C C ) = , ,..... r A B Mole Mole s m Units: Dimension: 3 Time Length 3 4
Chemical Reactions and Rate of Reactions: aA bB + + cC dD It is defined for each species in the chemical reaction, i.e. r r r r Negative Negative Positive Positive A B C D All species reaction rates in a certain reactions are interrelated through the stoichiometric ratios as follows: r r a r b r = = = C c A B D d Reaction rates can be only obtained experimentally. 5
Chemical Reactions and Rate of Reactions: Example 1: Nitrogen oxide is oxidize to nitrogen dioxide according to the following stoichiometric equation: + 2 2 NO O NO 2 2 If the rate of nitrogen dioxide was measured and found to be 4.0 mol/m3/s, what would be the rate of the two other species? 6
Reaction Rate Models Reaction Rate Models Power Law Models Complex Rate Model aA bB + + cC dD + 2 N O N O 2 2 2 = r k C C A A A B k C k C The concentrations of the individual reacting species, each of which is raised to a power product of concentration of N O + N O = N O r 2 2 1 2 O 2 Unit of the specific reaction rate: ( ) 1 n Concentration Time = = + , k n Reactions orders are determined by experimental observation 7
Batch Reactor: Definition: Batch Reactors are defined as reactors in which no flow of mass across the reactor boundaries, once the reactants have been charged. Schematic Representation of Batch Reactors: Stirrer Liquid Surface V Tank 8
Batch Reactor: Mode of Operation: Cleaning the reactor Unloading the reactor Loading the reactor Stopping the operation t = tf Adding the initiator t = 0 9
Batch Reactor: Characteristics of Batch Reactor: 1. Each batch is a closed system. 2. The total mass of each batch is fixed. 3. The volume or density of each batch may vary as reaction proceeds. 4. The energy of each batch may vary as reaction proceeds; heat exchanger may be provided to control temperature. 5. The reaction (residence) time for elements of the reacting fluid is the same. 6. The operation of the reactor is inherently unsteady-state; batch composition changes with respect to time. 7. At any time, the batch is uniform in composition, temperature because of the efficient and vigorous stirring 10
Continuous Stirred Tank Reactor (CSTR): Definition: Continuous Stirred Tank Reactors (CSTR) are defined to be flow reactors characterized by intense mixing so that the properties anywhere inside the reactor are exactly the same as that of the exist stream. Schematic Representation of CSTR: Stirrer This model can be used to: Liquid Surface V Input Rate 1. model a bed of catalyst i.e. fluidized-bed reactors. 2. Slurry column reactor 3. Polymerization reactors powder, bubble Output Rate 11
Continuous Stirred Tank Reactor (CSTR): Characteristics of Continuous Stirred Tank Reactor (CSTR): 1. The flow through the vessel(s), both input and out streams, is continuous but not necessary at a constant rate. 2. The system mass inside each vessel is not necessary fixed. 3. The volume or density of each batch may vary as reaction proceeds. 4. The energy of each batch may vary as reaction proceeds; heat exchanger may be provided to control temperature. 5. The reaction (residence) time for elements of the reacting fluid is the same. 6. The operation of the reactor may be steady state or unsteady-state. 7. The fluid properties are uniform in composition, temperature anywhere in the vessel because of the efficient and vigorous stirring 12
Plug Flow Reactors(PFR): Definition: Plug Flow Reactors (PFR) are defined to be flow reactors characterized by the absence of mixing in the direction of flow and absence of variation normal to the direction of flow. Schematic Representation of PFR: This model can be used to: 1. model a tubular- type reactors such as manufacturing reactor. ammonia 13
Plug Flow Reactors(PFR): Characteristics of Plug Flow Reactors(PFR): 1. The flow through the vessel(s), both input and out streams, is continuous but not necessary at a constant rate. 2. The system mass inside each vessel is not necessary fixed. 3. The density of the flowing system may vary in the direction of flow. 4. There is no axial mixing of fluid inside the reactor, composition changes along the flow direction. 5. There is complete radial mixing of fluid inside the reactor; uniform fluid properties along the direction normal to flow direction. 6. The energy may vary as reaction proceeds; heat exchanger may be provided to control temperature. 7. The reaction (residence) time for elements of the reacting fluid is the same. 8. The operation of the reactor may be steady state or unsteady-state. 14
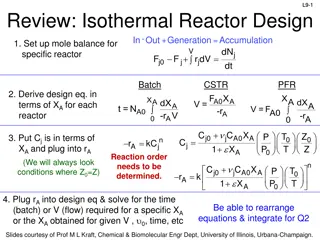
Example 2.2 The reaction described by A B is to be carried out in a flow reactor. Species A enters the reactor at a molar flowrate of 0.4 mol/s. Using the data provided: 1. Calculate the volume necessary to achieve 80% conversion in CSTR. 2. Shade the area that would correspond to the necessary volume. 3. Calculate the volume necessary to achieve 80% conversion in PFR. 4. Shade the area that would correspond to the necessary volume. 5. For two CSTR in series, 40% conversion is achieved in the first reactor. What is the volume of each of the two reactor necessary to achieve 80% overall conversion of the entering species A? 6. For two PFR in series, 40% conversion is achieved in the first reactor. What is the volume of each of the two reactor necessary to achieve 80% overall conversion of the entering species A? 15
Example 2.2 -rA (mol/m3 s) X 0.0 0.45 0.1 0.37 0.2 0.30 0.4 0.195 0.6 0.113 0.7 0.079 0.8 0.05 16
Example 2.2 -rA (mol/m3 s) 1/-rA (m3 s / mol) X 0.0 0.45 2.22 0.1 0.37 2.70 0.2 0.30 3.33 0.4 0.195 5.13 0.6 0.113 8.85 0.7 0.079 12.66 0.8 0.05 20.00 17
25.00 20.00 15.00 1/rA 10.00 5.00 0.00 0 0.2 0.4 Converstion, X 0.6 0.8 1 18
Thank you 19