Understanding the Rule of Mixtures in Composite Materials
The Rule of Mixtures (ROM) is a weighted method for predicting the properties of composite materials, such as fiber-reinforced polymers (FRP). This method relies on assumptions regarding the homogeneity and properties of fibers and matrices. By combining volume fraction and properties linearly, the
5 views • 23 slides
Overview of Herbicides Classification and Groups
Herbicides are classified based on their chemical nature into inorganic and organic types. Inorganic herbicides, such as arsenic acid and copper sulfate, do not contain carbon atoms, while organic herbicides, like glyphosate and 2,4-D, contain carbon atoms. The classification includes various groups
4 views • 15 slides
The Building Blocks of the Universe: Protons, Neutrons, Electrons, Atoms, and Beyond
The universe is composed of just seven different things - protons, neutrons, and electrons- the fundamental building blocks of matter. These particles combine to form atoms like hydrogen, helium, and carbon, which are essential components of our bodies. Understanding the structure and properties of
0 views • 37 slides
Tracing Carbon Atoms in Ecosystems: Understanding the Organic Matter Pyramid
Explore the journey of 500 carbon atoms through producers, herbivores, and carnivores in an ecosystem. Discover how carbon atoms move through photosynthesis, cellular respiration, and biosynthesis, ultimately contributing to the organic matter pyramid. Follow the pathways of carbon atoms as they cyc
0 views • 7 slides
Understanding Atoms: The Building Blocks of Life
Atoms are the fundamental units of matter, composed of protons, neutrons, and electrons. This article explores the structure of atoms, the atomic theory, and how atoms make up elements. Discover how changing the number of protons can create different elements, and learn about the periodic table and
5 views • 26 slides
Science Catch-Up Plan for Grades 7-9: April 2020
Explore a comprehensive catch-up plan for grades 7-9 in natural sciences compiled by Mr. Motsuenyane. The plan covers topics such as physical properties of materials, conducting experiments on strength, flexibility, boiling and melting points, conductivity, and environmental impacts. Grade 8 focuses
0 views • 9 slides
Understanding Temperature Effects on Donor and Acceptor Ionization in Semiconductors
Temperature plays a crucial role in the ionization of donor and acceptor atoms in semiconductors. In N-type semiconductors, the Fermi level lies below the conduction band, while in P-type semiconductors it lies above the valence band, with the position depending on temperature and impurity atoms. Do
1 views • 13 slides
Understanding Different Types of Chemical Bonds
Metallic bonds involve atoms giving up valence electrons to form an electron sea, covalent bonds entail electron sharing to fill outer orbitals, ionic bonds form when atoms with different electronegativities attract, Van der Waals bonds include London forces between atoms, and hydrogen bonds occur i
0 views • 6 slides
Understanding Atoms, Ions, and Isotopes in Chemistry
Atoms are neutral with equal protons and electrons. Ions are charged atoms resulting from gaining or losing electrons, while isotopes are atoms with varying numbers of neutrons. The atomic number always signifies the number of protons in an atom, unaffected by electron or neutron changes. Explore th
2 views • 5 slides
Understanding Valence Electrons in Atoms and the Periodic Table
Explore the concept of valence electrons in atoms, crucial for chemical bonding. Learn to define valence electrons, understand their role, and draw electron-dot structures for atoms. Practice identifying valence electrons in various elements and creating electron-dot structures in this educational c
1 views • 12 slides
Understanding Chemical Bonding and Stability in Atoms
Explore the significance of chemical bonds in providing stability to atoms through ionic and covalent bonding mechanisms. Learn about valence electrons, types of bonds, and why atoms form bonds for enhanced stability.
0 views • 16 slides
Understanding Nonelectrolytes in Solutions
Physical properties of substances are classified into colligative, additive, and constitutive properties. Colligative properties depend on the number of particles in a solution and are similar for different nonelectrolytes. Additive properties are based on the total contribution of atoms, while cons
1 views • 14 slides
Understanding Aldehydes and Ketones in Organic Chemistry
Aldehydes and ketones are compounds that contain carbonyl groups (>C=O). Aldehydes have the CO group linked to either two hydrogen atoms or one hydrogen atom and one alkyl or aryl group, while ketones have the CO group linked to two alkyl or aryl groups. The structure of the carbonyl group is charac
0 views • 26 slides
Understanding Atoms and Molecules: Basics and Models
Explore the world of atoms and molecules with a focus on distinguishing between them, understanding atomic structures through historic models like Bohr and Rutherford, and the modern atomic model. Discover how atoms form the basis of all matter and how they are organized within molecules.
0 views • 20 slides
The Journey of Atoms: From the Big Bang to Earth
The narrative explores the intriguing evolution of atoms from the Big Bang, where protons, neutrons, and electrons were born, to the formation of hydrogen and helium atoms. Over time, denser regions condensed to form stars, triggering nuclear fusion and creating heavier elements. This led to the exi
0 views • 24 slides
Overview of Herbicides Classification and Chemical Nature
Herbicides are classified based on their chemical nature into inorganic and organic herbicides. Inorganic herbicides do not contain carbon atoms, while organic herbicides contain carbon atoms. They are further categorized into 31 classes, each with distinct properties and modes of action. This class
0 views • 15 slides
Understanding Atoms and the Periodic Table
Explore the fundamentals of atoms and matter, including the conservation of matter, mixtures, pure substances, and the properties of metals, metalloids, and nonmetals. Discover the significance of valence electrons, the neutral state of atoms, and the classification of elements on the periodic table
0 views • 13 slides
Understanding Organic Chemistry: Carbon Atoms and Molecular Diversity
In organic chemistry, carbon atoms can form diverse molecules by bonding to four other atoms, leading to molecular complexity and diversity. The versatile nature of carbon allows for the formation of various carbon skeletons, contributing to the vast array of organic compounds. Hydrocarbons, consist
0 views • 12 slides
Understanding Electricity: Atoms, Circuits, and Charges
Discover the fundamentals of electricity through atoms, circuits, and electric charges. Learn how electrons flow, how circuits are created, and the key elements in a circuit. Explore conductors and insulators, and understand the basic properties of electric charges. Unveil the mysteries behind Coulo
0 views • 8 slides
Kaonic Atoms Research Workshop at Istituto Nazionale Fisica Nucleare
Dive into the world of kaonic atoms physics and the latest advancements in Silicon Drift Detectors technology for precision measurements at the Istituto Nazionale Fisica Nucleare workshops held in Frascati, Italy. Explore the research conducted on strange matter and strangeness studies in Italy and
0 views • 31 slides
Exploring the World of Atoms and Molecules
Dive into the realm of atoms, molecules, and ions as we uncover the key concepts behind Dalton's Atomic Theory, the discoveries of John Dalton and J.J. Thompson, and fundamental chemical laws. From the indivisibility of atoms to the Plum Pudding Model, this journey through chemistry's building block
0 views • 54 slides
Understanding Physical and Chemical Properties of Matter
Explore the distinction between physical and chemical properties of matter in Chapter 15, Section 2. Learn to classify properties such as color, flammability, odor, shape, taste, density, and more. Understand how physical properties can be observed without altering the substance's identity, while ch
0 views • 17 slides
Understanding Matter, Elements, and Molecules
Matter is anything with mass and volume, consisting of atoms. Elements are pure substances with one type of atom. Molecules are groups of bonded atoms. The content explains mass, volume, different forms of elements, allotropes, and compound properties like sodium chloride. It covers chemical symbols
0 views • 37 slides
Understanding Matter, Energy, Atoms, and Elements in Science
Explore the fundamental concepts of matter and energy, the structure of atoms, the properties of elements in the periodic table, and the classification of metals, nonmetals, and metalloids. Learn about compounds and mixtures and how they differ in composition and properties.
0 views • 22 slides
Photoproduction of Pionic Atoms at the Gamma Factory: Research Overview
Research conducted by V. V. Flambaum, J. Jin, and D. Budker at the Gamma Factory (GF) on photoproduction of pionic atoms is detailed in this content. It explores the formation of pionic atoms with negative pions orbiting the nucleus in a hydrogen-like system, emphasizing strong interaction effects a
0 views • 11 slides
Understanding Chemistry: Elements, Molecules, Compounds, and Mixtures Flow Chart
In the world of chemistry, elements are the building blocks represented on the Periodic Table, while molecules and compounds are formed by atoms bonding together. Mixtures, on the other hand, involve a combination of substances without chemical bonding. Physical and chemical properties define matter
0 views • 7 slides
Understanding Ions and Their Importance in Your Body
Neutral atoms have the same number of protons and electrons, with the charge of the nucleus always positive. The mass number is the sum of protons and neutrons. The number of neutrons can be calculated by subtracting the atomic number from the mass number. Ions are atoms with a positive or negative
0 views • 15 slides
Understanding Electron Arrangements in Atoms
Explore the concepts of electron arrangements in atoms through activities and discussions on valence and core electrons, shell models, and patterns in the periodic table. Discover how the number of electrons in shells relates to element properties and learn to create shell model diagrams. Dive into
0 views • 14 slides
Understanding Atoms: From Structure to Models
Explore the fundamental building blocks of matter - atoms. Discover what materials are made of, delve into atomic theories, examine Bohr models, and learn about the intricate components such as protons, neutrons, and electrons. Engage in a journey through the microscopic world of atoms and their sig
0 views • 34 slides
Understanding Matter: Solids, Liquids, Gases, and Fluids
Matter exists in various states - solid, liquid, gas, and fluid. Solids have atoms closely packed, liquids have more freedom but still cohesion, gases have atoms spread out, and fluids flow like liquids or gases. Mass density characterizes matter based on atom proximity. Gas pressure results from mo
0 views • 22 slides
Understanding Chemical Bonds and Molecular Geometry
Chemical bonds are the forces that hold atoms together, with valence electrons playing a crucial role. Ionic bonds involve complete electron transfer between metals and nonmetals, while covalent bonds see electrons being shared. Lewis dot diagrams help in visualizing the valence electrons of atoms,
0 views • 68 slides
Explore the Atoms Family in Matterville - A Digital Notebook Adventure
Discover the quirky Atoms Family in Matterville through a fun and educational digital notebook. Meet Perky Patty Proton, Nerdy Nelda Neutron, and Enraged Elliott Electron as they hang out at the Nucleus Arcade. Learn about their personalities, favorite activities, and how the balance of electrons an
0 views • 21 slides
Introduction to Chemistry and Matter
Understanding chemistry involves studying the properties of matter and its changes. Matter is anything that occupies space and has mass, composed of vibrating atoms. It includes examples like air, water, books, desks, and people, while non-examples are light, sound, thoughts, and emotions. Physical
0 views • 18 slides
Understanding Chemical Bonds: Covalent, Ionic, and Metallic
Explore the fascinating world of chemical bonds, including covalent bonds where atoms share electron pairs (e.g., water), ionic bonds where oppositely charged ions attract (e.g., sodium chloride), and metallic bonds formed between positively charged atoms sharing free electrons (e.g., copper wire).
0 views • 6 slides
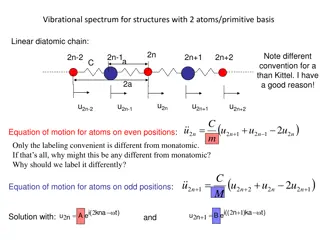
Vibrational Spectrum in Diatomic Chain Structures: Analysis and Eigenmodes
The vibrational spectrum of structures with two atoms in a linear diatomic chain is examined, focusing on the equation of motion for atoms at even and odd positions, phonon dispersion, transverse acoustic and optical modes, longitudinal eigenmodes in 1D, and extending the 1D model to 3D for phonon d
0 views • 20 slides
Understanding Atomic Absorption Spectroscopy in Analytical Chemistry
Atomic absorption spectroscopy (AAS) is a valuable technique for determining metal concentrations in samples. This method involves the absorption of light to measure gas-phase atoms, requiring steps like desolvation, vaporization, and volatilization to convert samples into atomic gas. The process ut
0 views • 18 slides
Understanding Atoms and Isotopes in Chemistry
Understanding atoms and isotopes is essential in chemistry. Atoms consist of protons, neutrons, and electrons that determine their properties. Isotopes are atoms of the same element with different atomic masses due to varying numbers of neutrons. Learning how to determine subatomic particles and ato
0 views • 17 slides
Energy Level Formation in Semiconductor Materials
Energy levels in isolated atomic structures form discrete levels which expand to bands in insulators, semiconductors, and conductors. Extrinsic materials like n-type and p-type are created by doping semiconductors with impurity atoms to alter conductivity. The n-type material involves adding pentava
0 views • 4 slides
Understanding Moles in Chemistry
Matter is composed of various particles, and chemists use the concept of moles as a unit of measure to quantify the number of particles in a substance. One mole is equal to 6.02 x 10^23 representative particles of a substance, known as Avogadro's number. Moles are versatile and applicable to differe
0 views • 25 slides
Three-Body Recombination in Ultracold Atoms: Studies and Observations
Investigating three-body recombination in ultracold atoms, this study explores the Efimov scenario, experimental setups with ultracold 7Li atoms, and the implications of three-body inelastic collisions. The research delves into universality windows, real molecule comparisons, and the loss rate from
0 views • 35 slides