Introduction to Chemistry and Matter
Understanding chemistry involves studying the properties of matter and its changes. Matter is anything that occupies space and has mass, composed of vibrating atoms. It includes examples like air, water, books, desks, and people, while non-examples are light, sound, thoughts, and emotions. Physical properties of matter include texture, color, hardness, and state of matter, while chemical properties relate to its ability to change into a different substance through reactions like burning or rusting.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
What is Chemistry? The study of the properties of matter and how matter changes
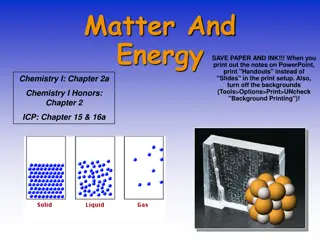
What is matter? Anything that takes up space and has mass Made up of atoms that vibrate due to kinetic energy
Desk? Yes!
Book? Yes!
Person? Yes!
Thoughts? I love science No
Water? Yes!
Light? No
Air? Yes!
Sounds? No
Emotions? No
Examples of Matter Book Desk Person Air Water
Non-examples of Matter Light Sound Thoughts Emotions
Physical Properties of Matter Characteristic of a substance that can be observed without changing it into another substance.
Texture Smell Taste Color Flexibility Hardness Weight Freezing point Melting point Boiling point Ability to dissolve (solubility) State of Matter (s, l, or g)
Chemical Properties of Matter Characteristic of a substance that describes its ability to change into a different substance
Ability to burn (Flammability) Ability to rust Ability to tarnish Ability to react with other substances (ie: acids)