Understanding Atoms, Ions, and Isotopes in Chemistry
Atoms are neutral with equal protons and electrons. Ions are charged atoms resulting from gaining or losing electrons, while isotopes are atoms with varying numbers of neutrons. The atomic number always signifies the number of protons in an atom, unaffected by electron or neutron changes. Explore the basic concepts of elements and their variations through ions and isotopes.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
Atoms Atoms are neutral Protons = Electrons There are special kinds of atoms.... Ions Isotopes
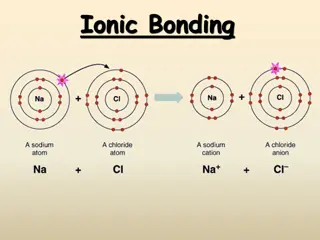
Ions An Ion is an atom that has gained or lost ELECTRONS, so it has an overall charge If an atom gains electrons, it s overall charge becomes negative. If an atom loses electrons, it s overall charge becomes positive. If an atom gains/loses electrons, its mass will not change.
Isotopes An Isotope is an atom that has gained or lost NEUTRONS If an atom gains/loses neutrons, charge is not affected. If an atom gains neutrons, its mass will increase. If an atom loses neutrons, its mass will decrease.
Atomic Number Because atoms can gain or lose electrons and neutrons to become ions or isotopes, the atomic number will ALWAYS identify the number of PROTONS an atoms has.