Understanding Valence Electrons in Atoms and the Periodic Table
Explore the concept of valence electrons in atoms, crucial for chemical bonding. Learn to define valence electrons, understand their role, and draw electron-dot structures for atoms. Practice identifying valence electrons in various elements and creating electron-dot structures in this educational chapter.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
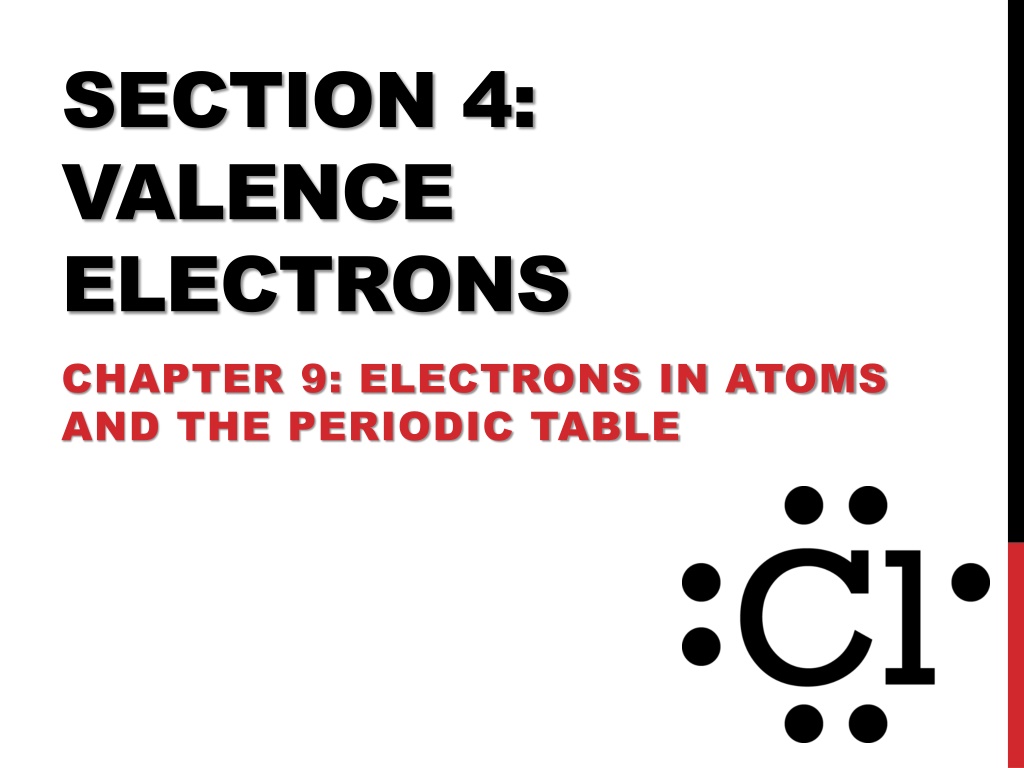
SECTION 4: VALENCE ELECTRONS CHAPTER 9: ELECTRONS IN ATOMS AND THE PERIODIC TABLE
Learning Goals Define valence electrons, and draw electron-dot structures representing an atom's valence electrons.
Valence Electrons Valence electrons are defined as electrons in the atom s outermost orbital These electrons are important because they are involved in chemical bonding. Electrons not in the outermost shell are called core electrons.
Valence Electrons How many valence electrons are in the following atoms? He O Na Cl
Valence Electrons How many valence electrons are in the following atoms? 1s22s22p6 1s22s22p63s23p64s23d104p4 [Kr]5s24d105p2 [Ne]3s23p3
Electron Dot Structure Electron-dot structure consists of the element s symbol representing the nucleus, surrounded by dots representing the element s valence electrons.
Electron Dot Structure Write the electron-dot structures for the following elements: He O Na Cl
Electron Dot Structure Write the electron dot structures for the following elements: 1s22s22p3 1s22s22p63s23p64s23d104p1 [Kr]5s24d105p5 [Ne]3s2