Exploring the World of Atoms and Molecules
Dive into the realm of atoms, molecules, and ions as we uncover the key concepts behind Dalton's Atomic Theory, the discoveries of John Dalton and J.J. Thompson, and fundamental chemical laws. From the indivisibility of atoms to the Plum Pudding Model, this journey through chemistry's building blocks illuminates how elements combine and react to form compounds.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Chapter 2 Atoms, Molecules, and Ions
Three Fundamental Chemical Laws Law of definite proportion (Proust): A given compound always contains exactly the same proportion of elements by mass. (It has a constant composition) Law of multiple proportions (Dalton): When two elements form a series of compounds, the ratios of the masses of the second element that combine with 1 gram of the first element can always be reduced to small whole numbers.
What ?! Water has 8 g of oxygen per 1 g of hydrogen. Hydrogen peroxide has 16 g of oxygen per 1 g of hydrogen. 16/8 = 2/1 Small whole number ratios.
John Dalton ) A Quaker schoolmaster (became a teacher at the age of 12) who studied all sciences, but made his greatest contributions in chemistry. Developed Atomic Theory and Law of Multiple Proportions. Atomic Theory helped to explain many of the observations that scientists were making. Law of Multiple Proportions helped to explain that 2 elements could combine to form more than 1 compound; for example CO and CO2.
Daltons Atomic Theory 1. Elements are made up of atoms which are indivisible. 2. Atoms of the same element are identical. Atoms of different elements are different. 3. Compounds are formed when atoms combine. Each compound has a specific number and kind of atom. 4.Chemical reactions are a rearrangement of atoms. Atoms are not created or destroyed.
Indivisible? Well, Dalton did this work in the early 1800 s. We know now that atoms are composed of protons, neutrons and electrons. Dalton didn t know about them they hadn t been discovered yet! HOWEVER, the atom is the smallest part of an element that retains the properties of that element. So an atom of gold is still gold and is different from an atom of carbon.
JJ Thompson In 1897, Thompson discovered the electron. Electrons are negatively charged and have almost no mass at all, compared to a proton. Thompson revised Dalton s model of the atom with one of his own, called the Plum Pudding Model.
Experiments to determine what an atom was ! J.J. Thompson used cathode ray tubes in his experiment The cathode ray tube (CRT) is a vacuum tube containing one or more electron guns, and a fluorescent screen used to view images
Thomsons Experiment - + Voltage source
Thomsons Experiment Voltage source - + Passing an electric current makes a beam appear to move from the negative to the positive end.
Thomsons Experiment Voltage source + - By adding an electric field, he found that the moving pieces were negative
Thompsons Plum Pudding Model Found the electron. Couldn t find positive (for a while). Said the atom was like plum pudding. A bunch of positive stuff, with the electrons able to be removed. Plum Pudding is a British dessert in which plums are scattered more or less randomly throughout a cake (the pudding).
Ernest Rutherford The Plum Pudding Model wouldn t last long, because one of JJ s former students did some experiments that forced the model to be revised again. Rutherford was from New Zealand, and like his mentor, Thompson, also won a Nobel Prize for his work. His work was the famous gold foil experiments, where he was researching alpha particles. As sometimes happened, Rutherford didn t set out to discover what he actually did.
Rutherfords Experiment Used uranium to produce alpha particles. Aimed alpha particles at gold foil by drilling hole in lead block. Since the mass is evenly distributed in gold atoms alpha particles should go straight through. Used gold foil because it could be made atoms thin.
Florescent Screen Lead block Uranium Gold Foil
Because, he thought the mass was evenly distributed in the atom.
How he explained it Atom is mostly empty + Small dense, positive piece at center. Alpha particles are deflected by it if they get close enough.
Neils Bohr Rutherford s nuclear model only really lasted for about 3 years, before Neils Bohr (who, oh by the way, also won a Nobel Prize for this) revised it again. Bohr asked a question: if the electrons are rotating around the nucleus, why don t they run out of energy. As they did, they would come closer and closer, attracted by the opposite charge of the nucleus, and eventually collapse onto the nucleus, destroying the atom in the process. This doesn t happen, and Bohr answered why. His model is usually called the Planetary model, because in his model, electrons orbit the nucleus much as our planets orbit the Sun. Soccer goalie on Denmark s 1908 Olympic team AND a Nobel Prize winner!!
Bohrs Planetary Model But the electrons don t just orbit anywhere. They actually exist in orbits that Bohr called energy levels. Each energy level has a certain amount of energy. Electrons can move to a higher energy level by gaining energy. Or they can drop to a lower energy level by losing (or emitting) energy.
Energy Levels An energy level is a region around the nucleus where an electron is likely to be moving. The first energy level (n = 1) has the lowest energy. It is called the ground state. Things in nature prefer to be in the lowest possible energy state.
The Modern Model of the Atom Many scientists (Louis DeBroglie, Max Planck, Albert Einstein, Erwin Schroedinger, and many others) worked on the model of the atom. Actually, they weren t working on the model of the atom. They were just working on interesting scientific problems. But they all made contributions to our current understanding of the atom. Quantum wave mechanics is the modern model of the atom. By the early 1930s, it had been born. It s the model we still use today.
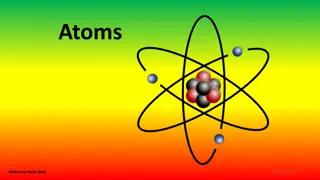
Modern View The atom is mostly empty space. Two regions Nucleus- protons and neutrons. Electron cloud- region where you might find an electron.
Sub-Atomic Particles Z - atomic number = number of protons determines type of atom. A - mass number = number of protons + neutrons. Number of protons = number of electrons if neutral.
Symbols 23 A X Na 11
Chemical Bonds The forces that hold atoms together. Covalent bonding - sharing electrons. Makes molecules. Chemical formula- the number and type of atoms in a molecule. C2H6- 2 carbon atoms, 6 hydrogen atoms,
Molecular Models Structural formula shows the connections, but not necessarily the shape. There are also other model that attempt to show three dimensional shape. Ball and stick.
Ions and Polyatomic Ions Atoms or groups of atoms with a charge. Cations- positive ions - created by losing electrons(s). Anions- negative ions - created by gaining electron(s). Ionic bonding- held together by the opposite charges. Ionic solids are called salts.
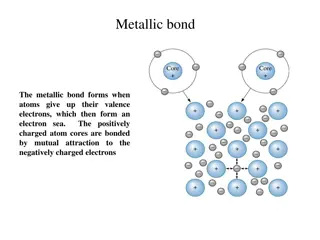
Metals Conductors Lose electrons Malleable and ductile
Nonmetals Brittle Gain electrons Covalent bonds
Inner Transition Metals
Naming compounds Three types Binary Ionic Type I Compounds Binary Ionic Type II Compounds Binary Polyatomic Type II Compounds Binary Covalent Compounds
Binary Ionic Type I Compounds (+) cation and a (-) anion Use the name of the first element and then take the root name of the second element and add the suffix -ide to it. Examples: NaCl ...sodium chloride Li3N .Lithium, Nitride
Remember !!! + 1 cations + 2 cations - 1 anions - 2 anions - 3 anions Group 1 . Group 2 . Group 17 Group 16 .... Group 15 .
Binary Ionic Type II Compounds The metals in Type II compounds are capable of forming more than one type of cation. Transition Metals (Group 3 - 12) The charge on the metal ion must be given in the name. Examples: FeCl2 iron (II) Chloride FeCl3 iron (III) Chloride Zn, Al, Ag only form one cation so it is not necessary to use a number after them.
Binary Ionic Compounds Type II Polyatomic Ions You have to use the special names that you memorized this past summer. Example NH4NO3 . Ammonium Nitrate Na2SO4 . Sodium Sulfate Fe ( NO3) . Iron (III) Nitrate
Binary Covalent Compounds Type III Formed between two nonmetals Name the first element using the full name Name the second element and give it an - ide on the end. Use prefixes to show the number of atoms present. Examples N2O .. Dinitrogen Monoxide CO2 .. Carbon Dioxide N2O5 .... Dinitrogen Pentoxide
Naming Covalent Compounds Prefixes 1 mono 6 hexa 2 di 7 septa 3 tri 8 octo 4 tetra 9 nono 5 penta 10 deca
Acids Substances that produce H+ions when dissolved in water. All acids begin with H. Two types of acids: Oxyacids Non-oxyacids
Naming acids If the formula has oxygen in it write the name of the anion, but change ate to -ic acid ite to -ous acid Watch out for sulfuric and sulfurous H2CrO4 HMnO4 HNO2
Naming acids If the acid doesn t have oxygen add the prefix hydro- change the suffix -ide to -ic acid HCl H2S HCN