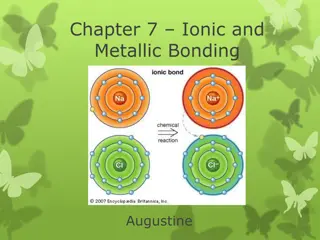
Understanding Ionic and Metallic Bonding: Valence Electrons, Octet Rule, and Ion Formation
Explore the essential concepts of ionic and metallic bonding, focusing on valence electrons, electron dot structures, the octet rule, cations, anions, and ion formation. Discover how atoms achieve stability through electron transfer, and learn to write electron configurations for various ions.
9 views • 52 slides
Understanding Total Solids and Total Suspended Solids in Water
Learn about total solids and total suspended solids in water, including their definitions, implications on water quality and aquatic life, and factors affecting their levels. Total solids refer to matter suspended or dissolved in water, while total suspended solids are small solid particles that can
7 views • 12 slides
Understanding the States of Matter: Solids, Liquids, and Gases
Matter is anything that occupies space and has mass, consisting of tiny particles like atoms and molecules. Solids have closely packed particles, liquids have less densely packed particles that can flow, and gases have spread out particles. Solids retain their shape, liquids take the shape of their
7 views • 11 slides
Understanding Crystal Structures and Types of Solids in Materials Science
Crystal structures in materials science involve the arrangement of atoms in solids, with examples of crystalline and amorphous solids like metals and glass. Explore concepts like energy packing, definitions of crystalline materials, unit cells, BCC and FCC lattices, HCP structures, and Miller Indice
10 views • 12 slides
Understanding Ionic and Metallic Bonding in Chemistry
Explore the concepts of ionic and metallic bonding in chemistry through discussions on valence electrons, electron dot structures, the octet rule, cations, anions, and more. Dive into the world of ions and electron configurations to understand how atoms achieve stability through the gain or loss of
3 views • 62 slides
Understanding Ionic and Covalent Bonding in Chemistry
Ionic bonding involves the transfer of electrons between a metal and a non-metal to form a giant lattice structure, like in sodium chloride and lithium oxide. Covalent bonding, on the other hand, occurs between non-metals, resulting in giant covalent structures or simple molecules. Examples such as
4 views • 79 slides
Philosophy of Thales: The Ancient Wisdom of the Ionic School
Thales of Miletus, a key figure in the Ionic School of philosophy, is considered the father of philosophy. He believed that water was the principle of all things and made significant contributions to mathematics and astronomy. Thales's wisdom and engineering feats, such as diverting the river Halys,
0 views • 18 slides
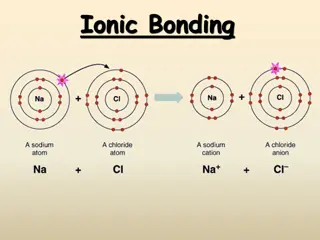
Understanding Chemical Bonds and Ionic Compounds
Ionic bonds are formed when atoms transfer electrons to achieve stable electron configurations, resulting in the creation of ions with positive or negative charges. Metals are good conductors due to their ability to easily lose electrons. The charges of ions depend on the number of valence electrons
0 views • 49 slides
Understanding Ionic and Metallic Bonding in Chemistry
Explore the concepts of ions, electron dot structures, the octet rule, cations, and anions in Chapter 7. Learn how elements achieve stability through electron configurations, and practice writing electron dot structures and naming ions. Understand the differences between cations and anions and how t
1 views • 52 slides
Calculus I Lecture #13: Volumes of Solids and Solids of Revolution
Exploring the concepts of finding volumes of solids using integrals, by slicing solid objects with parallel planes and calculating cross-sectional areas. Examples include calculating the volume of a pyramid and a curved wedge. The method of solids of revolution using the disk method is also discusse
0 views • 12 slides
Exploring Properties of Matter: Solids, Liquids, and Gases
Dive into the characteristics of solids, liquids, and gases through engaging visuals and key vocabulary. Understand the behavior of particles in different states of matter and learn about the properties that define each state. Explore examples of solids, liquids, and gases with a focus on their uniq
0 views • 19 slides
Chemistry Concepts: Valence Electrons, Ion Charges, and Ionic Compounds
Explore various key concepts in chemistry such as valence electrons in magnesium, Lewis Dot structure for silicon, charges on ions like strontium, formation of ions to achieve noble-gas electron configuration, elements forming ions with specific charges, and the octet rule. Learn about the character
1 views • 48 slides
Understanding Ionic Bonding and Lattice Energy
Explore the world of ionic bonding through images and explanations. Learn how electrons are transferred to form ions, the arrangement of ions in a crystal lattice, and the concept of lattice energy in ionic compounds. Discover the formation of formula units, examples of bond pairs, and the significa
1 views • 18 slides
Rejuvenate with Ionic Foot Detox in New Jersey
Experience the benefits of ionic foot detox at Zen Health & Wellness Center in New Jersey. Detoxify, relax, and rejuvenate for improved overall well-being. Book your session today.\nFor more information:\nCall us: 8564620579\nMail us: ZENHEALTHANDWEL
4 views • 8 slides
Understanding Ion-Pair Chromatography (IPC): Theory and Applications
Ion-Pair Chromatography (IPC) involves adding ionic surfactants to a reversed-phase Chromatography system to affect retention and selectivity of ionic compounds. Developed by Dr. Gordon Schill, IPC is crucial for resolving hydrophilic samples and controlling selectivity in separations. The theory in
7 views • 18 slides
Chemical Bonding and Compound Formulas: Understanding Ionic vs. Covalent Bonds
Explore the differences between ionic and covalent bonds, learn about ionic compounds held by electromagnetic attractions, understand molecular compounds with shared electrons, and grasp the naming conventions for ions. Discover how molecular formulas and formula units represent atoms in compounds.
0 views • 56 slides
Understanding Protein Solubility: Effects of pH, Ionic Strength, and Salting Out
Exploring the factors influencing the solubility of proteins, including pH levels, ionic strength, and salting out techniques. This study delves into the isoelectric point of proteins and the impact of salt addition on solubility, aiming to enhance comprehension in the field of protein biochemistry
0 views • 14 slides
Chemistry: Naming Compounds and Writing Formulas
Understand compounds, chemical formulas, and how to write ionic formulas using the Swap 'n Drop Method. Learn about types of compounds - ionic, covalent, and acids, and practice writing formulas for various elements. Follow rules, naming flow charts, and partner activities to enhance your understand
0 views • 13 slides
Overview of Wastewater Treatment Units and Processes
Screening units with screens and racks remove coarse solids, comminutors reduce the size of suspended solids, and grit chambers remove sand and metal fragments from wastewater to protect downstream equipment and processes. The floatation tank removes grease, while different designs of grit chambers
5 views • 15 slides
Understanding Wastewater Treatment Process Through Images
Screening devices and grit chambers play crucial roles in sewage treatment plants to remove coarse solids and suspended solids like sand and metal fragments. The aerated grit chamber helps separate organic and inorganic solids efficiently, ensuring the effectiveness of biological treatment and mecha
3 views • 9 slides
Understanding Ions, Ionic Bonds, and Ionic Compounds
Ions are charged particles formed by gaining or losing electrons, leading to the formation of ionic bonds between positively charged cations and negatively charged anions. The octet rule guides electron configurations, and the periodic table helps predict ion formation. Ionic bonding involves electr
0 views • 13 slides
Exploring Solids and Properties of Matter
Dive into the world of solids and properties of matter, from seating arrangements to classroom objectives. Discover the different phases of matter, investigate properties of solids, liquids, and gases, and explore the interactions between particles. Engage in hands-on activities and learning experie
0 views • 105 slides
Understanding Cardiac Action Potential: Ionic Basis and Excitability in Cells
Explore the complex processes underlying cardiac action potential, from ionic equilibrium to resting membrane potential and excitability in cardiac cells. Learn about the critical thresholds, equilibrium potentials, and gradients that regulate the electrical activity of the heart. Discover the intri
0 views • 68 slides
Understanding Ionic Compound Formulation and Nomenclature
Ionic compounds consist of cations and anions combined in simple ratios to balance charges. The cross technique helps determine the correct ratio of ions. Reduction of ratios may be necessary in certain cases. Formulation and naming examples are provided to illustrate these concepts.
1 views • 12 slides
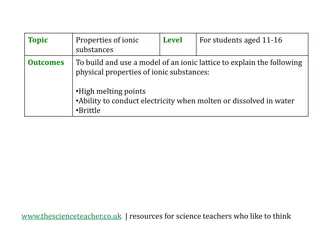
Exploring Physical Properties of Ionic Substances through Model Building
Engage students aged 11-16 in an interactive activity to understand the physical properties of ionic substances such as high melting points, ability to conduct electricity, and brittleness. By building a model of an ionic lattice for sodium chloride and explaining how the structure relates to these
0 views • 5 slides
Understanding Chemical Bonding and Atomic Properties
Explore the formation of ionic and covalent bonds, electron configurations of ions, and molecular geometry. Learn about ionic compound formation, atomic properties like effective nuclear charge, atomic size, ionization energy, and electron affinity. Discover the essential concepts of cations formati
1 views • 73 slides
Understanding States of Matter and Kinetic Theory
Matter is anything that occupies space and has mass, existing in solid, liquid, gas, and plasma states. The states of matter depend on the arrangement and motion of atoms. Solids have fixed shapes, liquids take the shape of their container, and gases fill the volume of their container. The Kinetic T
0 views • 18 slides
Understanding Electrolytes and Ionic Equilibrium in Chemistry
Electrolytes play a crucial role in conducting electricity through ionization in aqueous solutions, with examples of strong and weak electrolytes explained. The concept of ionic equilibrium, degree of dissociation, and distinction between non-electrolytes are also covered in this comprehensive overv
0 views • 21 slides
Understanding Primary Bonding in Solids: Importance and Types
Exploring the fundamental concept of bonding in solids, this lecture delves into primary bonding, which includes covalent, ionic, and metallic bonds. The discussion highlights how different types of bonds impact the properties of materials such as metals, ceramics, and polymers. By understanding the
0 views • 19 slides
Understanding Matter: Solids, Liquids, Gases, and Fluids
Matter exists in various states - solid, liquid, gas, and fluid. Solids have atoms closely packed, liquids have more freedom but still cohesion, gases have atoms spread out, and fluids flow like liquids or gases. Mass density characterizes matter based on atom proximity. Gas pressure results from mo
0 views • 22 slides
Understanding Ionic and Molecular Compounds in Chemistry
Discover the fundamental concepts of ionic and molecular compounds in chemistry with insights into the nature of elements, formation of compounds, and properties of ions. Explore the differences between ionic and covalent bonds, positive and negative ions, as well as examples of common everyday comp
0 views • 54 slides
Vision-Based Particle Analysis Techniques for Solids and Liquids Measurement
Solids and Liquids in Liquids and Solids in Liquid Measurement are performed by JM Canty, Inc. Their innovative vision-based particle analysis techniques involve dynamic imaging with high-intensity light sources and cameras to measure particle size, shape, and concentration in various liquid systems
0 views • 22 slides
Understanding Chemical Bonds: Covalent, Ionic, and Metallic
Explore the fascinating world of chemical bonds, including covalent bonds where atoms share electron pairs (e.g., water), ionic bonds where oppositely charged ions attract (e.g., sodium chloride), and metallic bonds formed between positively charged atoms sharing free electrons (e.g., copper wire).
0 views • 6 slides
Understanding Ionic Bonding and Octet Rule in Chemistry
Understanding the concept of ionic bonding and octet rule in chemistry is essential for grasping how atoms combine to form molecules through sharing or exchanging electrons. This process involves the formation of positive and negative ions held together by electrostatic attraction, leading to the cr
0 views • 12 slides
Understanding Ionic Bonding and Lattice Energy in Chemistry
Chemical bonds play a crucial role in holding atoms together in molecules. This course explores the concept of chemical bonding, focusing on ionic bonds and lattice energy. Topics covered include the different types of chemical bonds, such as electrovalent and coordinate bonds, as well as the models
0 views • 22 slides
Understanding Solid State Chemistry: Principles and Classification of Solids
Solid State Chemistry (CHEM 422) explores the principles and concepts governing the synthesis, structure, bonding, reactivity, and properties of solid state materials. The course delves into crystalline vs. amorphous solids, highlighting categories like ionic, molecular, metallic, and covalent solid
0 views • 24 slides
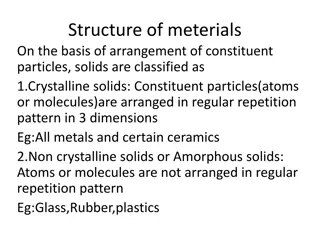
Crystal Structure and Classification of Solids Based on Particle Arrangement
Solids are classified into crystalline and non-crystalline based on the arrangement of constituent particles. Crystalline solids exhibit a regular repetitive pattern in three dimensions, while non-crystalline solids lack such order. The structure of metals includes body-centered cubic (BCC), face-ce
0 views • 56 slides
Understanding Ionic Bonding and Naming in Chemistry
Exploring the fundamentals of ionic bonding, naming conventions, and the Octet Rule in chemistry. Learn about Lewis structures, formation of ionic compounds, and the role of valence electrons in determining chemical properties. Discover how elements gain or lose electrons to achieve a full outer ene
0 views • 24 slides
Understanding Bonding in Ionic, Covalent, and Metallic Compounds
Explore the concepts of ionic, covalent, and metallic bonding through an investigation conducted by Vanderbilt Student Volunteers for Science. Learn about the different types of bonding, properties of ionic and molecular compounds, and the conductivity of metals. Discover the importance of determini
0 views • 15 slides
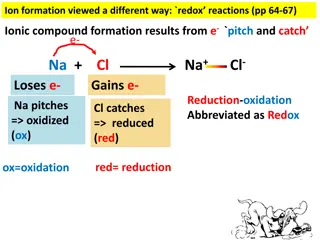
Understanding Redox Reactions and Ionic Compound Formation
Explore the concept of redox reactions through the process of ion formation, where elements lose or gain electrons to create ionic compounds. Learn about oxidation (ox) and reduction (red) in chemical reactions, and how to identify which elements lose or gain electrons based on charge changes. Disco
0 views • 14 slides