Chemistry Concepts: Valence Electrons, Ion Charges, and Ionic Compounds
Explore various key concepts in chemistry such as valence electrons in magnesium, Lewis Dot structure for silicon, charges on ions like strontium, formation of ions to achieve noble-gas electron configuration, elements forming ions with specific charges, and the octet rule. Learn about the characteristics of ionic compounds, including cation charges and net charges of compounds like sodium sulfide and calcium fluoride.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
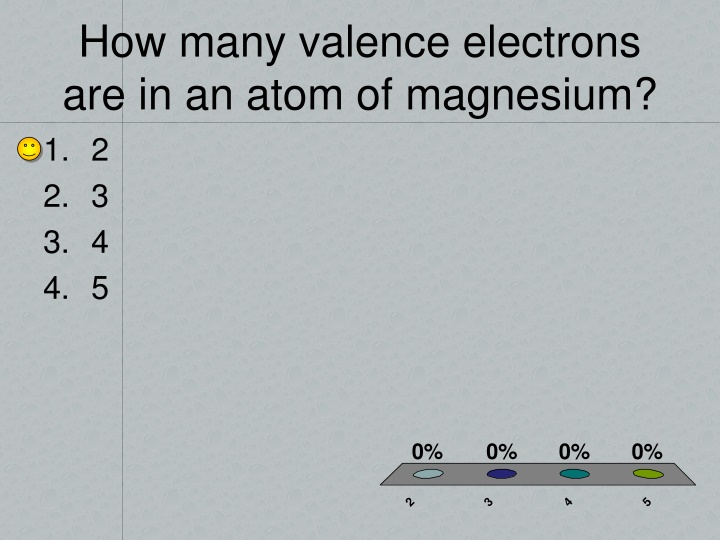
How many valence electrons are in an atom of magnesium? 1. 2 2. 3 3. 4 4. 5 0% 0% 0% 0% 2 3 4 5
How many dots around a silicon atom in a Lewis Dot? 1. 2 2. 4 3. 6 4. 8 0% 0% 0% 0% 2 4 6 8
What is the charge on the strontium ion? 1. 2- 2. 1- 3. 1+ 4. 2+ 0% 0% 0% 0% 1+ 2+ 2- 1-
What is the formula of the ion formed when potassium achieves noble-gas electron configuration? 1. K2+ 2. K1+ 3. K1- 4. K2- 0% 0% 0% 0% K1- K2- K2+ K1+
Which of the following elements forms an ion with a charge of -1? 1. fluorine 2. hydrogen 3. potassium 4. sodium 0% 0% 0% 0% sodium fluorine potassium hydrogen
How many electrons does nitrogen gain in order to achieve a noble-gas electron configuration? 1. 1 2. 2 3. 3 4. 4 0% 0% 0% 0% 1 2 3 4
How does oxygen obey the octet rule when reacting to form compounds? 1. It gains electrons. 2. It gives up electrons. 3. It does not change its number of electrons. 4. Oxygen does not obey the octet rule. 0% 0% 0% 0% It gains electrons. It gives up electrons. Oxygen does not ob.. It does not change it...
What is the charge on each cation in the ionic compound sodium sulfide? 1. 1- 2. 1 3. 2 4. 2- 0% 0% 0% 0% 1 2 -1 -2
What is the net charge of the ionic compound calcium fluoride? 1. 2- 2. 1- 3. 0 4. 1 0% 0% 0% 0% 0 1 2- 1-
Which of the following is true about an ionic compound? 1. It is a salt. 2. It is held together by ionic bonds. 3. It is composed of anions and cations. 4. all of the above 0% 0% 0% 0% It is a salt. all of the above It is held together by ... It is composed of ani...
How many valence electrons are transferred from the calcium atom to iodine in the formation of the compound calcium iodide? 1. 0 2. 1 3. 2 4. 3 0% 0% 0% 0% 0 1 2 3
What is the formula for the ionic compound sodium nitride? 1. Na3N 2. NaN3 3. Na2N2 4. NaN 0% 0% 0% 0% NaN Na3N NaN3 Na2N2
What is the formula for aluminum oxide? 1. Al2O3 2. AlO3 3. Al3O2 4. AlO 0% 0% 0% 0% AlO3 Al2O3 Al3O2 AlO
What is the formula for potassium sulfide? 1. KS2 2. K2S2 3. K2S 4. KS 0% 0% 0% 0% KS K2S KS2 K2S2
How does titanium obey the octet rule when reacting to form compounds? 1. It gains electrons. 2. It gives up electrons. 3. It does not change its number of electrons. 4. Calcium does not obey the octet rule. 0% 0% 0% 0% It gains electrons. It gives up electrons. Calcium does not ob.. It does not change it...
What is the charge of the anion in the ionic compound calcium sulfide? 1. -1 2. 1 3. 2 4. -2 0% 0% 0% 0% 1 2 -1 -2
What is the charge of the cation in the ionic compound CaS? 1. 1- 2. 1 3. 2- 4. 2+ 0% 0% 0% 0% 1 2+ 1- 2-
What is the charge on each ammonium ion in Rb3N? 1. 3- 2. 2- 3. 1- 4. 1+ 5. 2+ 0% 0% 0% 0% 0% 1+ 2+ 3- 2- 1-
What is the net charge of the ionic compound calcium fluoride? 1. 2- 2. 1- 3. 0 4. 1 0% 0% 0% 0% 0 1 2- 1-
What is the formula for the ionic compound sodium nitrite? 1. Na3N3 2. Na3N 3. Na2N3 4. NaN3 0% 0% 0% 0% Na3N NaNO3 Na3NO3 Na2NO3
What is the formula of iron(III) oxide? 1. FeO3 2. FeO 3. Fe2O3 4. Fe3O2 0% 0% 0% 0% FeO3 FeO Fe2O3 Fe3O2
What is the formula for potassium sulfide? 1. K2S 2. K2S4 3. K4S2 4. K(SO4)2 0% 0% 0% 0% K2SO4 K2(SO)4 K(SO4)2 K2(SO4)2
What is the formula of the ion formed when potassium achieves noble-gas electron configuration? 1. K2+ 2. K1+ 3. K1- 4. K2- 0% 0% 0% 0% K1- K2- K2+ K1+
Name the following compound: CaCl2 1. Calcium dichloride 2. Calcium (II) chloride 3. Calcium chloride 4. Calcium chlorine 0% 0% 0% 0% Calcium chloride Calcium chlorine Calcium dichloride Calcium (II) chloride
What is the correct formula for magnesium chloride? 1. MgCl 2. Mg2Cl 3. MgCl2 4. Mg2Cl2 0% 0% 0% 0% MnCl MnCl2 Mn2Cl Mn2Cl2
What is the correct formula for sodium phosphate? 1. NaP 2. NaPO4 3. Na3PO4 4. Na(PO4)3 0% 0% 0% 0% NaP NaPO4 Na3PO4 Na(PO4)3
How many valence electrons are in an atom of magnesium? 1. 2 2. 3 3. 4 4. 5 0% 0% 0% 0% 2 3 4 5
How many valence electrons are in a silicon atom? 1. 2 2. 4 3. 6 4. 8 0% 0% 0% 0% 2 4 6 8
How does calcium obey the octet rule when reacting to form compounds? 1. It gains electrons. 2. It gives up electrons. 3. It does not change its number of electrons. 4. Calcium does not obey the octet rule. 0% 0% 0% 0% It gains electrons. It gives up electrons. Calcium does not ob.. It does not change it...
What is the electron configuration of the calcium ion? 1. 1s22s22p63s2 2. 1s22s22p63s23p6 3. 1s22s22p63s23p64s2 4. 1s22s22p63s23p24s2 0% 0% 0% 0% 1s22s22p63s2 1s22s22p63s23p6 1s22s22p63s23p64s2 1s22s22p63s23p24s2
What is the electron configuration of the sulfide ion? 1. 1s22s22p63s23p6 2. 1s22s22p63s23p2 3. 1s22s22p63s23p4 4. 1s22s22p63s6 0% 0% 0% 0% 1s22s22p63s6 1s22s22p63s23p6 1s22s22p63s23p2 1s22s22p63s23p4
What is the charge on the strontium ion? 1. 2- 2. 1- 3. 1+ 4. 2+ 0% 0% 0% 0% 1+ 2+ 2- 1-
What is the formula of the ion formed when potassium achieves noble-gas electron configuration? 1. K2+ 2. K1+ 3. K1- 4. K2- 0% 0% 0% 0% K1- K2- K2+ K1+
Which of the following elements forms an ion with a charge of -1? 1. fluorine 2. hydrogen 3. potassium 4. sodium 0% 0% 0% 0% sodium fluorine potassium hydrogen
How many electrons does nitrogen gain in order to achieve a noble-gas electron configuration? 1. 1 2. 2 3. 3 4. 4 0% 0% 0% 0% 1 2 3 4
How does oxygen obey the octet rule when reacting to form compounds? 1. It gains electrons. 2. It gives up electrons. 3. It does not change its number of electrons. 4. Oxygen does not obey the octet rule. 0% 0% 0% 0% It gains electrons. It gives up electrons. Oxygen does not ob.. It does not change it...
What is the electron configuration of the oxide ion (O)? 1. 1s22s2 2. 1s22s22p2 3. 1s22s22p4 4. 1s22s22p6 0% 0% 0% 0% 1s22s2 1s22s22p2 1s22s22p4 1s22s22p6
What is the charge on the cation in the ionic compound sodium sulfide? 1. -1 2. 1 3. 2 4. -2 0% 0% 0% 0% 1 2 -1 -2
What is the net charge of the ionic compound calcium fluoride? 1. 2- 2. 1- 3. 0 4. 1 0% 0% 0% 0% 0 1 2- 1-
Which of the following is true about an ionic compound? 1. It is a salt. 2. It is held together by ionic bonds. 3. It is composed of anions and cations. 4. all of the above 0% 0% 0% 0% It is a salt. all of the above It is held together by ... It is composed of ani...
How many valence electrons are transferred from the calcium atom to iodine in the formation of the compound calcium iodide? 1. 0 2. 1 3. 2 4. 3 0% 0% 0% 0% 0 1 2 3
What is the formula for the ionic compound sodium nitride? 1. Na3N 2. NaN3 3. Na2N2 4. NaN 0% 0% 0% 0% NaN Na3N NaN3 Na2N2
What is the formula unit of aluminum oxide? 1. Al2O3 2. AlO3 3. Al3O2 4. AlO 0% 0% 0% 0% AlO3 Al2O3 Al3O2 AlO
What is the formula for potassium sulfide? 1. KS2 2. K2S2 3. K2S 4. KS 0% 0% 0% 0% KS K2S KS2 K2S2
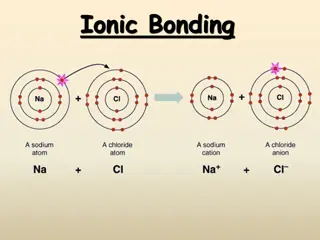
An ionic bond is a bond between ____. 1. a cation and an anion 2. valence electrons and cations 3. the ions of two different metals 4. the ions of two different nonmetals 0% 0% 0% 0% a cation and an anion the ions of two differe.. the ions of two differe.. valence electrons an...
Write the formula for the compound barium oxide.
Write the formula for the compound rubidium phosphide.
Write the formula for the compound boron chloride.