Understanding Ionic Bonding and Naming in Chemistry
Exploring the fundamentals of ionic bonding, naming conventions, and the Octet Rule in chemistry. Learn about Lewis structures, formation of ionic compounds, and the role of valence electrons in determining chemical properties. Discover how elements gain or lose electrons to achieve a full outer energy level and form stable compounds.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
Ionic Bonding and Naming Chapter 7 and 9 1
SC1 Students will analyze the nature of matter and its classifications. SC1.b. Identify substances based on chemical and physical properties. SC1.c. Predict formulas for stable ionic compounds (binary and tertiary) based on balance of charges. SC1.d. Use IUPAC nomenclature for both chemical names and formulas: SC1.d.1 Ionic compounds (Binary and tertiary) SC1.d.3 Acidic compounds (Binary and tertiary) SC3.e. Compare and contrast types of chemical bonds (i.e. ionic, covalent). SC3.b. Use the orbital configuration of neutral atoms to explain its effect on the atom s chemical properties. 2
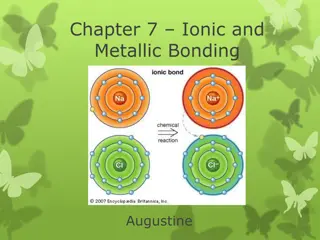
What do we already know? Protons determine the IDENTINTY of the element Valance Electrons determine the CHEMICAL properties of an element. Valance electrons are the electrons in the OUTER ENERGY level For representative elements look at the group number to determine the number of valance electrons Elements lose or gain electrons to achieve a full outer energy level (full OCTET) Metals form CATIONS by losing electrons Nonmetals form ANIONS by gaining electrons 3
The Octet Rule Octet rule stated that in forming compounds atoms tend to achieve the electron configuration of a noble gas. An octet is a set of eight electrons Atoms of metals tend to lose their valence electrons leaving a complete octet in the next- lowest energy level. Atoms of some nonmetals tend to gain electrons or to share electrons with another nonmetals to achieve a complete octet. 4
Lewis Structure Electron dot structures (Lewis dot structure) are diagram that show the valence electrons at dots. Each valance electron is represented with a dot Put one dot on each side of the symbol before putting two on one side. Examples 1.Carbon 2.Calcium 3.Chlorine 4.Argon 4 valance e- 2 valance e- 7 valance e- 8 valance e- C Cl Ca Ar 5
Formation of Ionic Compounds Ionic compounds are compounds composed of cations and anions. Although they are compounds of ions, ionic compounds are electrically neutral. Ionic bonds are the electrostatic forces that hold ions together in ionic compounds. They occur due to the transfer of electrons Chemical formula shows the kinds and numbers of atoms in the smallest representative unit of a substance. Formula unit is the lowest whole-number ratio of ions in an ionic compound 6
Formula Writing 1. When writing formulas the MOST METALIC element is written first Ionic bonds occur between METALS and NONMETALS so the metal is ALWAYS written FIRST. 2. Determine the ion that the elements will form 3. Balance charges Can switch charges and reduce if necessary Or can use the following equation: # of metal x charge of metal + # of nonmetal x charge of nonmetal = 0 atoms atoms 7
Formula Writing Practice 1: Oxygen and Sodium 1. Sodium is metal so it MUST be written first 2. Na forms +1 ion and O forms -2 ion 3. Na+1 O-2 switching charges gives Na2O (# metal) +1 +(#nonmetal) -2 = 0 solve (2) +1 +(1) -2 = 0 gives Na2O NOTE: The subscript of 1 is NOT written Practice 2: Nitrogen and Aluminum 1. Aluminum is metal so it MUST be written first 2. Al forms +3 ion and N forms -3 ion 3. Al+3 N-3 switching charges gives AlN (must reduce) (# metal) +3 +(#nonmetal) -3 = 0 solve (1) +3 +(1) -3 = 0 gives AlN 8
Formula Writing Practice 3: Calcium and Carbon 1. Calcium is metal so it MUST be written first 2. Ca forms +2 ion and C forms -4 ion 3. Ca+2 C-4 switching charges gives Ca2C (# metal) +2 +(#nonmetal) -4 = 0 solve (2) +2 +(1) -4 = 0 gives Ca2C Practice 4: Barium and Phosphate (PO4-3) 1. Barium is metal so it MUST be written first 2. Ba forms +2 ion and PO4 is a -3 ion 3. Al+2 PO4-3 switching charges gives Al3(PO4)2 (# metal) +2 +(#nonmetal) -3 = 0 solve (3) +2 +(2) -3 = 0 gives Al3(PO4)2 MUST USE parenthesis to show having 2 phosphate molecules. 9
Ionic Naming Type 1 metals Type 1 metals are metals that form only 1 oxidation state. They are found in groups 1, 2, & 13 in addition to Zn+2, Cd+2, and Ag+1 1. Determine the Cation and the Anion 2. If the cation is from a representative element write its name 3. The anion is a: a. Polyatomic ion write it s special name b. Single nonmetal element write its root name followed by ide 4. write both parts of name side by side 10
Ionic Naming Type 1 Example 1: Ca3N2 1. Calcium is the cation, nitrogen is anion 2. Calcium stays calcium 3. Nitrogen is NOT a polyatomic so it becomes Nitride 4. Ca3N2 is called calcium nitride Example 1: Ca3(PO4)2 1. Calcium is the cation, phosphate is anion 2. Calcium stays calcium 3. Phosphate is a polyatomic so it s name is phosphate 4. Ca3(PO4)2 is called calcium phosphate 11
Ionic Naming Type 1 Practice 1. KCl 2. AlCl3 3. Ca2C 4. InN 5. Rb3PO3 6. Al(OH)3 7. In2(SO3)3 8. (NH4)3Br Practice 1. Potassium chloride 2. Aluminum chloride 3. Calcium carbide 4. Indium nitride 5. Rubidium phosphite 6. Aluminum hydroxide 7. Indium sulfite 8. ** Ammonium bromide 12
Ionic Naming Type 2 metals Most transition metals have the ability to borrow electrons from other orbitals and can form ions with different charges. Metals in group 14 also have multiple oxidation states. +2 or +4 Example: Iron can from a +3 or +4 cation, copper can from a +2 or +1 ion Not ALL transition metal do this but MOST do so when we name the compound we have to state the charge of the metal ion EXCEPTIONS: three transition metals that you MUST memorize the following: Zn+2, Cd+2, Ag+1 as they do NOT need roman numerals 13
Ionic Naming Type 2 Metals 1. Determine the Cation and the Anion 2. If the cation is from a transition element write its name followed by a roman numeral to show the charge of the metal ion. 3. The anion is a: a. Polyatomic ion write it s special name b. Single nonmetal element write its root name followed by ide 4. write both parts of name side by side 14
Ionic Naming Type 2 Common Roman numerals you MUST KNOW 1. I 6. VI ** 2. II 7. VII 3. III 8. VIII 4. IV ** 9. IX 5. V 10. X ** commonly confused by students 15
Ionic Naming Type 2 Example 1: FeO 1. Fe is cation and O is anion 2. since Oxygen has a -2 charge Fe MUST have a +2 so it is Iron (II) 3. O is NOT a polyatomic so it becomes Oxide 4. FeO is Iron (II) Oxide Example 2: Fe2O3 1. Fe is cation and O is anion 2. since Oxygen has a -2 charge Fe MUST have a +3 so it is Iron (III) 3. O is NOT a polyatomic so it becomes Oxide 4. FeO is Iron (III) Oxide 16
Ionic Naming Type 2 Example 3: FePO4 1. Fe is cation and PO4 is anion 2. since PO4 has a -3 charge Fe MUST have a +3 so it is Iron (III) 3. PO4 is a polyatomic so it is phosphate 4. FeO is Iron (III) Phosphate Example 4: Ag2O 1. Ag is cation and O is anion 2. Ag is an exception and only forms a +1 ion so is Silver 3. O is NOT a polyatomic so it becomes Oxide 4. Ag2Ois Sliver Oxide Remember Zn+2, Cd+2, Ag+1 do NOT need roman numerals 17
Ionic Names to formula 1. Use the name to determine the ions of the elements (or polyatomic) in compound 2. Write the ions for each element 3. Balance charges using either method(reduce if necessary) Chemical formulas for COMPOUNDS do NOT have charges!! The number of atoms MUST be shown as a subscript. REMEMBER the size and the shape of the letters matter when writing chemical formulas: COS and CoS are two different things 18
Ionic Names to Formulas Examples 1. Strontium Sulfide Sr+2 S-2 SrS 2. Magnesium Cyanide Mg +2 CN-1 Mg(CN)2 3. Potassium Phosphide K+1 P+3 K3P Examples 4. Zinc Oxide Zn+1 O-2 Zn2O 5. Cobalt (II) Oxide Co+2 O-2 CoO 6. Manganese (IV) Sulfate Mn+4 S-2 MnS2 19
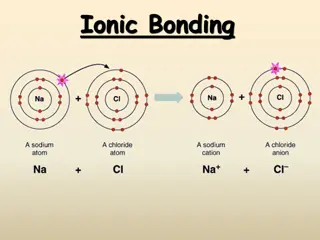
Formation of Ionic Compounds Metals lose their electrons to nonmetals The opposite charges attract and form an ionic bond Na + Cl Na + Cl Na+1 + Cl-1 NaCl name is: sodium chloride Mg + S Mg + S Mg+2 + S-2 MgS name is: Magnesium Sulfide 20
Properties of Ionic Compounds Ionic compounds form by the TRANSFER of electrons Most ionic compounds are crystalline solids at room temperature. Ions in the crystals are arranged in repeating three- dimensional patterns. The large attractive forces result in very stable structures Ionic compounds generally have high melting points. Ionic compounds can conduct an electric current when melted or dissolved in water The ion movement allows electricity to flow between electrodes 21
Metallic Bonds and Metallic Properties The valence electrons of metal atoms can be modeled as a sea of electrons. Metallic bonds consist of the attraction of the free- floating valence electrons for the positively charged metal ions. the sea-of-electrons models explains many physical properties of metals metals are good conductors of electric current because electrons can flow freely in them metals are malleable (can be hammered or forced into shapes.) metals are ductile (can be drawn into wires) 23
Alloys metal atoms are arranged in very compact and orderly patterns alloys are mixtures composed of two or more elements, at least of one which is metal alloys are important because their properties are superior to those of their components elements. Bronze alloy is made of copper and iron Steel alloys are made of iron and carbon with additional elements. 24