Understanding Chemical Bonds and Ionic Compounds
Ionic bonds are formed when atoms transfer electrons to achieve stable electron configurations, resulting in the creation of ions with positive or negative charges. Metals are good conductors due to their ability to easily lose electrons. The charges of ions depend on the number of valence electrons in an element. By understanding ion charges and electron configurations, we can predict the behavior of elements in forming compounds.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
CHAPTER 6 CHEMICAL BONDS Jennie L. Borders
WARM-UP MAR. 11 1. What is an ionic bond? 2. What charge does an aluminum ion have? 3. Why are metals good conductors of electricity and heat?
SECTION 6.1 IONIC BONDS When the highest occupied energy level of an atom is filled with electrons, the atom is stable and not likely to react. The noble gases have stable electron configurations with eight valence electrons (or two in the case of helium).
STABLE ELECTRON CONFIGURATIONS The chemical properties of an element depend on the number of valence electrons. An electron dot diagram is a model of an atom in which each dot represents a valence electron.
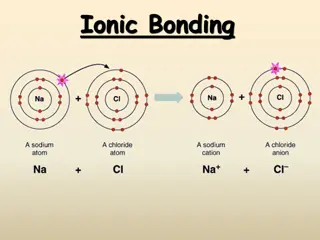
IONIC BONDS Some elements achieve stable electron configurations through the transfer of electrons between atoms. An atom that has a net positive or negative charge is called an ion.
IONS An ion with a negative charge is an anion. Anions like the Cl-ion are named by adding the suffix ide to the element name. (Ex. Cl-= chloride) An ion with a positive charge is a cation. The name of a cation is the same as the element name. (Ex. Na+= sodium)
ION CHARGES The charges that an ion has are based on the number of valence electrons that an element has. All of the elements in the same group have the same charge.
ION CHARGES Is it easier for lithium to gain 7 more electrons or lose 1 electron? What charge would lithium have? lose 1 electron +1
ION CHARGES Is it easier for beryllium to gain 6 more electrons or lose 2 electrons? What charge would beryllium have? lose 2 electrons +2
ION CHARGES Is it easier for boron to gain 5 more electrons or lose 3 electrons? What charge would boron have? lose 3 electrons +3
ION CHARGES Is it easier for carbon to gain 4 more electrons or lose 4 electrons? lose or gain 4 electrons What charge would carbon have? +4 or -4
ION CHARGES Is it easier for nitrogen to gain 3 more electrons or lose 5 electrons? What charge would nitrogen have? gain 3 electrons -3
ION CHARGES Is it easier for oxygen to gain 2 more electrons or lose 6 electrons? What charge would oxygen have? gain 2 electrons -2
ION CHARGES Is it easier for fluorine to gain 1 more electron or lose 7 electrons? What charge would fluorine have? gain 1 electron -1
ION CHARGES Would neon want to gain or lose electrons? What charge would neon have? No, it has a full energy level 0
ION CHARGES Since elements in the same group have the same number of valence electrons, they all have the same charge.
FORMATIONOF IONIC BONDS A chemical bond is the force that holds atoms or ions together as a unit. An ionic bond is the force that holds cations and anions together. An ionic bond forms when electrons are transferred from one atom to another.
FORMATIONOF IONIC BONDS When an ionic bond is formed, electrons are transferred until each atom has a full outer energy level.
IONIC COMPOUNDS Compounds that contain ionic bonds are ionic compounds, which can be represented by chemical formulas. A chemical formula is a notation that shows what elements a compound contains and the ratio of the atoms or ions of these elements in the compound.
CRYSTAL LATTICES A chemical formula for an ionic compound tells you the ratio of the ions in the compound. Solids whose particles are arranged in a lattice structure are called crystals.
PROPERTIESOF IONIC COMPOUNDS Ionic compounds tend to have high melting points (above 300oC). Ionic compounds are poor conductors in the solid state, but they can conduct heat or electricity when they are melted. Ionic compounds are brittle, so they shatter when struck by a hammer. The properties of ionic compounds can be explained by the strong attractions among ions within a crystal lattice.
SECTION 6.1 ASSESSMENT 1. When is an atom least likely to react? 2. Describe one way an element can achieve a stable electron configuration. 3. What characteristic of ionic bonds can be used to explain the properties of ionic compounds? 4. What will the ratio of ions be in any compound formed from a Group 1 metal and a Group 17 nonmetal?
SECTION 6.1 ASSESSMENT 5. Why do ionic compounds include at least one metal? 6. Based on their chemical formulas, which of these compounds is not likely to be an ionic compounds: KBr, SO2, or FeCl3?
WARM-UP MAR. 12 1. What charge do the elements in Group 16 have? 2. What are 3 properties of an ionic compound? 3. Name the following ions: a. Ca+2 b. Br-
SECTION 6.2 COVALENT BONDING A covalent bond is a chemical bond in which two atoms share a pair of valence electrons. When two atoms share one pair of electrons, the bond is called a single bond.
MOLECULESOF ELEMENTS A molecule is a neutral group of atoms that are joined together by one or more covalent bonds. The attractions between the shared electrons and the protons in each nucleus hold the atoms together in a covalent bond. Many nonmetal elements exist as diatomic molecules. Diatomic means two atoms. They are H2, N2, O2, F2, Cl2, Br2, and I2.
MULTIPLE COVALENT BONDS When two atoms share two pairs of electrons, the bond is called a double bond. When two atoms share three pairs of electrons, the bond is called a triple bond.
SECTION 6.2 ASSESSMENT 1. What attractions hold atoms together in a covalent bond? 2. Which of these elements does not bond to form molecules: oxygen, chlorine, neon, or sulfur? 3. Based on their electron dot diagrams, what is the formula for the covalently bonded compound of nitrogen and hydrogen?
WARM-UP MAR. 13 What is a covalent bond? List the 7 diatomic elements. What is a molecule? 1. 2. 3.
SECTION 6.3 NAMING COMPOUNDSAND WRITING FORMULAS The name of an ionic compound must distinguish the compound from other ionic compounds containing the same elements. The formula of an ionic compound describes the ratio of the ions in the compound.
BINARY IONIC COMPOUNDS A compound made from only two elements is a binary compound. When naming an ionic compound the name of the metal (cation) does not change and the name of the nonmetal (anion) has the suffix ide. Ex. MgBr2 = magnesium bromide
METALWITH MULTIPLE IONS Many transition metals form more than one type of ion. When a metal forms more than one ion, the name of the ion contains a roman numeral to indicate the charge of the ion. Ex: Fe+2 = iron (II) Fe+3 = iron (III)
POLYATOMIC IONS A covalently bonded group of atoms that has a positive or negative charge and acts as a unit is a polyatomic ion.
WRITING FORMULAS FOR IONIC COMPOUNDS Place the symbol of the cation first, followed by the symbol of the anion. Use subscripts to show the ratio of the ions in the compound. Because all compounds are neutral, the total charges on the cations and anions must add up to zero.
CROSSING CHARGES To balance the charges in an ionic compound, you can cross the charges if they are not the same.
DESCRIBING MOLECULAR COMPOUNDS The name and formula of a molecular compound describe the type and number of atoms in a molecule of the compound. Molecular compounds only contain nonmetals.
NAMING MOLECULAR COMPOUNDS The name of the first element is the same. The name of the second element ends in the suffix -ide. Prefixes tell the number of atoms of each element. A prefix is not used when the first element only has 1 atom. Ex. CO2 = carbon dioxide
PREFIXES 1 = mono 2 = di 3 = tri 4 = tetra 5 = penta 6 = hexa 7 = hepta 8 = octa 9 = nona 10 = deca
WRITING MOLECULAR FORMULAS Write the symbols for the elements in the order the elements appear in the name. The prefixes indicate the number of atoms of each element in this molecule. Ex: diphosphorus pentoxide = P2O5
SECTION 6.3 ASSESSMENT 1. What does the formula of an ionic compound describe? 2. What do the name and formula of a molecular compound describe? 3. What suffix is used to indicate an anion? 4. Why are Roman numerals used in the names of compounds that contain transition metals? 5. What is a polyatomic ion?
SECTION 6.3 ASSESSMENT 6. How is it possible for two different ionic compounds to contain the same elements? 7. How many potassium ions are needed to bond with a phosphate ion? 8. What are the name of these ionic compounds: LiCl, BaO, and Na3N? 9. Name the molecular compounds with these formulas: N2O7 and CO. 10. What is the formula for the ionic compound formed from potassium and sulfur?
SECTION 6.4 THE STRUCTURE OF METALS In a metal, valence electrons are free to move among the atoms, so the cations are surrounded by a sea of electrons. A metallic bond is the attraction between a metal cation and the shared electrons that surround it.
METALLIC BONDS The cations in a metal form a lattice that is held in place by strong metallic bonds between the cations and the surrounding valence electrons. The more valence electrons an atom can contribute to the shared pool, the stronger the metallic bond will be.
PROPERTIESOF METALS The mobility of electrons within a metal lattice explains the fact that metals are good conductors and malleable.
ALLOYS An alloy is a mixture of two or more elements that have metallic properties.
SECTION 6.4 ASSESSMENT 1. What holds metal ions together in a metal lattice? 2. What characteristic of a metallic bond explains some of the properties of metals? 3. Explain why the metallic bonds in some metals are stronger than the bonds in other metals. 4. Why are metals good conductors of electric current? 5. Can two different elements form a metallic bond together?