Exploring Physical Properties of Ionic Substances through Model Building
Engage students aged 11-16 in an interactive activity to understand the physical properties of ionic substances such as high melting points, ability to conduct electricity, and brittleness. By building a model of an ionic lattice for sodium chloride and explaining how the structure relates to these properties, students will grasp fundamental concepts in chemistry. Encourage critical thinking using props and models to represent ions, bonds, and lattice structures.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
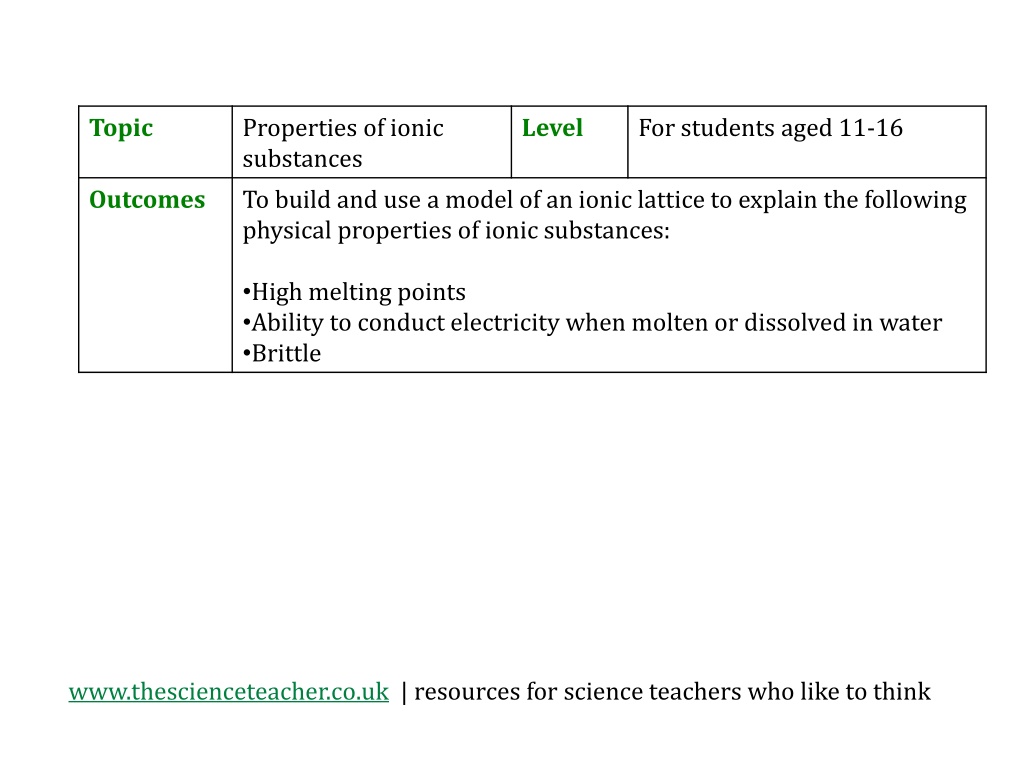
Topic Properties of ionic substances To build and use a model of an ionic lattice to explain the following physical properties of ionic substances: Level For students aged 11-16 Outcomes High melting points Ability to conduct electricity when molten or dissolved in water Brittle www.thescienceteacher.co.uk | resources for science teachers who like to think
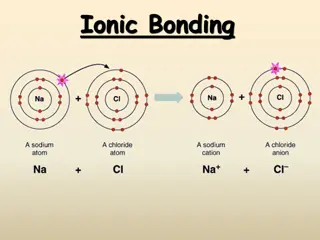
Aim: You are going to build a model of an ionic lattice for sodium chloride using straws and plasticine. It must be built in 3D. Things to think about: What will you use to represent the ions? What will you use to represent the ionic bonds between the ions? How will you represent the difference between the chloride and the sodium ions? Think about their electronic structure. How will you show that a lattice is a repeating structure?
Physical Property Further Information You are going to use your model to explain each of the physical properties to the class. Ionic substances have high melting points. There are strong forces of attraction between the oppositely charged ions. These ionic bonds have to be broken when a substance melts. This requires lots of energy. You will need to think about: 1. What are you going to say during your explanation? Prepare a brief script. 2. How are you going to use your model to help your explanation? Solid ionic substances do not conduct electricity. Charges have to be able to flow for a substance to conduct electricity. Molten ionic substances are able to conduct electricity. When we melt an ionic substance the ionic bonds are broken. Each explanation should last 3 minutes. You cannot use notes. You can use other props to adapt and improve your model. Ionic substances can dissolve in water and the solution formed will conduct electricity. Water molecules surround the ions in the lattice. This releases energy and breaks the ionic bonds allowing the ions to move.
When we hit a lump of sodium chloride with a hammer it breaks up into lots of little pieces. This is because the force applied by the hammer causes the arrangement of the ions to change. Can you figure out what might happen when the hammer hits a lattice of sodium chloride? Represent what you think is happening using your model.