Understanding Ionic and Molecular Compounds in Chemistry
Discover the fundamental concepts of ionic and molecular compounds in chemistry with insights into the nature of elements, formation of compounds, and properties of ions. Explore the differences between ionic and covalent bonds, positive and negative ions, as well as examples of common everyday compounds. Gain a deeper understanding of electron transfer, stability, and the octet rule in chemical bonding.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
Course Lecturer: Assist.Prof. Dr. Altijana Hromi -Jahjefendi
In nature almost all elements are found in combination Only noble gases do not combine (very stable) A compound is composed of two or more elements Can be ionic and molecular
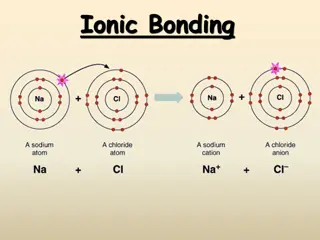
Ionic compound One or more electrons are transferred from metals to nonmetals Forms positive and negative ions The attraction between these ions is ionic bond Everyday examples: kitchen salt NaCl, baking soda NaHCO3, mineral supplements etc. Precious and semiprecious gemstones (minerals) are also ionic compounds Sapphire and rubies are made of Al2O3 with impurities
Molecular compounds Consists of two or more nonmetals that share one or more valence electrons Resulting molecules are held by covalent bonds Examples: water H2O, carbon dioxide CO2, alcohol, antibiotics etc.
Ions: Transfer of Electrons Compounds form when electrons are transferred or shared to give stable electron arrangement to the atoms! Ionic or covalent bond Gain, lose or share valence electrons to acquire an octet (8 valence electrons) Tendency to be in stable electron arrangement is octet rule
Positive Ions: Loss of Electrons Ions electrical charges Form when atoms lose or gain electrons Metals Positively charged ions of metals cations A metal ion is named by its element name Examples: Na+, Mg2+, etc.
Negative Ions: Gain of Electrons By gaining electrons, a nonmetal atom forms a negatively charged ion Anion The name is formed by using the first syllable of its element name followed by ide Example: Chloride
Ionic Charges from Group Numbers Using group numbers in the periodic table to determine the charges for the ions of the representative elements Group 1A lose one electron to form ions with 1+ charge Example: Na+ Group 2A lose two electrons to form ions with 2+ charge Example: Mg2+
Groups 5-7 are gaining electrons to form ions with negative charge
Three ways to find the valence electrons: http://www.wikihow.com/Find-Valence-Electrons
Link to Health Some ions are important in the body Physiological and metabolic function Food provides body with ions important in regulating body functions
Ionic compounds - Review Consist of positive and negative ions Held together by ionic bons Positive ions formed by metals Negative ions formed by nonmetals
Properties of ionic compounds Properties of an compound are very different from those of the original elements Example: NaCl Sodium (Na) is soft, shiny, very reactive metal Chlorine (Cl) is yellow-green poisonous gas Upon reaction they produce NaCl ordinary table salt Hard, white, crystalline substance
Cl-ions are larger Na+ions are smaller Arranged in 3D structure in which Na+ions occupy the space between the Cl-ions Every Na+ion is surrounded by six Cl-ions and vice versa Many strong attractions between the positive and negative ions Accounts for the high melting points
Chemical formulas of ionic compounds The chemical formula of a compound represents the symbols and subscripts in the lowestwhole-number ratio of the atoms or ions. Thus, the total amount of positive charge is equal to the total amount of negative charge. Na atom loses its one valence electron to form Na+, and one Cl atom gains one electron to form a Cl- ion Formula indicates that the compound has charge balance
Subscripts in Formulas Example: Mg and Cl Mg loses 2 electrons (Mg2+) Two Cl atoms gain one electron to form two Cl-ions Two Cl-ions are needed to balance positive charge of Mg2+ Gives formula MgCl2 Subscript 2 indicates that 2 Cl-ions are needed for charge balance
Naming Ionic Compounds Name of metal ion is the same as its element name The name of nonmetal ion is first syllable + ide Subscripts are not used, they are understood
Polyatomic ions An ionic compound may also contain a polyatomic ion as one of its cations or anions. A polyatomic ion is a group of covalently bonded atoms that has an overall ionic charge. Most polyatomic ions consist of a nonmetal such as phosphorus, sulfur, carbon, or nitrogencovalently bonded to oxygen atoms. Almost all the polyatomic ions are anions with charges of 1-, 2-, or 3-. Only onecommon polyatomic ion, NH4 +, has a positive charge..
Names of Polyatomic Ions Names of most of them end in ate (nitrate, sulfate) When a related ion has one less oxygen atom ending ite (nitrite, sulfite) Hydroxide ion (OH-) and cyanide ion (CN-) are exceptions to this naming pattern
Writing formulas for compounds containing polyatomic ions No polyatomic ion exists by itself It must be associated with ions of opposite charge To write correct formula same rules as for writing simple ionic compounds The total negative and positive charge must equal zero
Naming Ionic Compounds Containing Polyatomic Ions First we write the positive ion (usually a metal) Then we write the name for the polyatomic ion No prefixes are used
Summary Ions Octet/ octet rule Cations Anions Ionic charges from group numbers Properties of ionic compounds Chemical formulas of ionic compounds Naming ionic compounds Polyatomic ions
Molecular compounds Contains two or more nonmetals that form covalent bons Valence electrons are shared covalent bond Atoms sharing electrons form a molecule
Formation of a covalent bond Hydrogen atoms (2 H) In each hydrogen atom, the single electron is held in its orbital by its attraction to the proton in the nucleus. 1 + + When two hydrogen atoms approach each other, the electron of each atom is also attracted to the proton in the other nucleus. 2 + + The two electrons become shared in a covalent bond, forming an H2 molecule. 3 + + Hydrogen molecule (H2)
Covalent bond Sharing a pair of valence electrons by two atoms Two or more atoms held together by covalent bonds constitute a molecule Occurs in non-metals Can be single, double and triple bond
Difference between single, double and triple bond Single bond= a pair of shared electrons (hydrogen H2) Double bond= sharing a two pairs of valence electrons (oxygen O2) Triple bond= sharing three pairs of valence electrons (nitrogen)
Names and formulas of Molecular Compounds The first nonmetal in the formula is named by its element name Second nonmetal is named using first syllable of its element name + ide When a subscript indicates two or more atoms of an element, a prefix is shown in front of its name
Prefixes are needed Different compounds can be formed from the same two nonmetals Example: carbon and oxygen Carbon monoxide (CO) and carbon dioxide (CO2) Prefix indicates the number of oxygen atoms
Electronegativity and Bond Polarity Electronegativity- the attraction of a particular kind of atom for the electrons in a covalent bond The more electronegative an atom the more strongly it pulls shared electrons toward itself Covalent bond between two atoms of the same element are equally electronegative
In some cases, more electronegative atoms strip electrons away from their bonding partners Typical example is Na and Cl Na has 1 electron in valence shell and Cl has 7 Lone valence electron of Na is transferred to the chlorine atom and both atoms have their valence shell completed
Bonding capacity corresponds to the number of covalent bonds th atom can form Also called valence Equal to the number of unpaired electrons in the valence shell
Polarity of Bonds The difference in electronegativity values of two atoms can be used to predict the type of bond 2 types: 1. polar covalent bond 2. non-polar covalent bond
In a polar covalent bond The atoms have different electronegativities Share the electrons unequally Vary in polarity
In a nonpolar covalent bond The atoms have same electronegativities Share the electron equally H2 or O2
Dipoles and Bond Polarity The polarity of a bond depends on the difference in the electronegativity values of its atoms In polar bond shared electrons are attracted to the more electronegative atom; makes them partially negative Atom with the lower electronegativity becomes partially positive; lack of the electrons at that atom
A bond becomes more polar as the electronegativity difference increases Polar covalent bond with separation of charges is called a dipole The positive and negativeends of the dipole are indicated by the lowercase Greek letter delta with a positive or negative sign, + and -
Polarity of molecules 2 types of covalent bonds 2 types of molecule polarities: 1. polar 2. nonpolar
Nonpolar molecules All the bonds are nonpolar Or the polar bonds cancel each other out because of symmetrical arrangement H2, Cl2, CH4 contain only nonpolar bonds CO2 is linear, has two equal polar covalent bonds whose dipoles point in opposite directions Dipoles cancel out making CO2 nonpolar molecule
Polar molecules One end of the molecule is more negatively charged than the other end Dipoles from the individual polar bonds do not cancel each other HCl has one covalent bond that is polar so the molecule is polar
Attractive forces in compounds In gases the interaction between particles is minimal In solids and liquids there are sufficient interactions between particles to hold them close together Dipole-dipole attractions Hydrogen bonding Dispersion forces
Dipole-dipole Attractions Between the positive end of one molecule and negative end of another Partially positive atom of one molecule attracts partially negative atom in another molecule HCl
Hydrogen Bonds Hydrogen atoms bonded to electronegative atom of nitrogen, oxygen or fluorine Form especially strong dipole-dipole attractions hydrogen bond Between partially positive H atom of one molecule and partially negative (N,O,F) atom in another molecule Strongest types of attractive forces Major factor in the formation and structure of biological molecules (proteins and DNA)