Understanding Ionic Bonding and Octet Rule in Chemistry
Understanding the concept of ionic bonding and octet rule in chemistry is essential for grasping how atoms combine to form molecules through sharing or exchanging electrons. This process involves the formation of positive and negative ions held together by electrostatic attraction, leading to the creation of various ionic compounds. Metal monatomic cations play a crucial role in this bonding process. Explore the forces that hold atoms together to form functional units, whether through ionic or covalent bonds, and delve into examples of different ionic compounds such as magnesium chloride, sodium oxide, and aluminum sulfide.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
Ga Standards Students know atoms combine to form molecules by sharing electrons to form covalent or metallic bonds or by exchanging electrons to form ionic bonds. Students know salt crystals, such as NaCl, are repeating patterns of positive and negative ions held together by electrostatic attraction.
Bonds Forces that hold groups of atoms together and make them function as a unit. Ionic bonds transfer of electrons Covalent bonds sharing of electrons
The Octet Rule Ionic Compounds Ionic compounds form so that each atom, by gaining or losing electrons, has an octet of electrons in its highest occupied energy level. Metals lose electrons to form positively-charged cations Nonmetals gains electrons to form negatively- charged anions
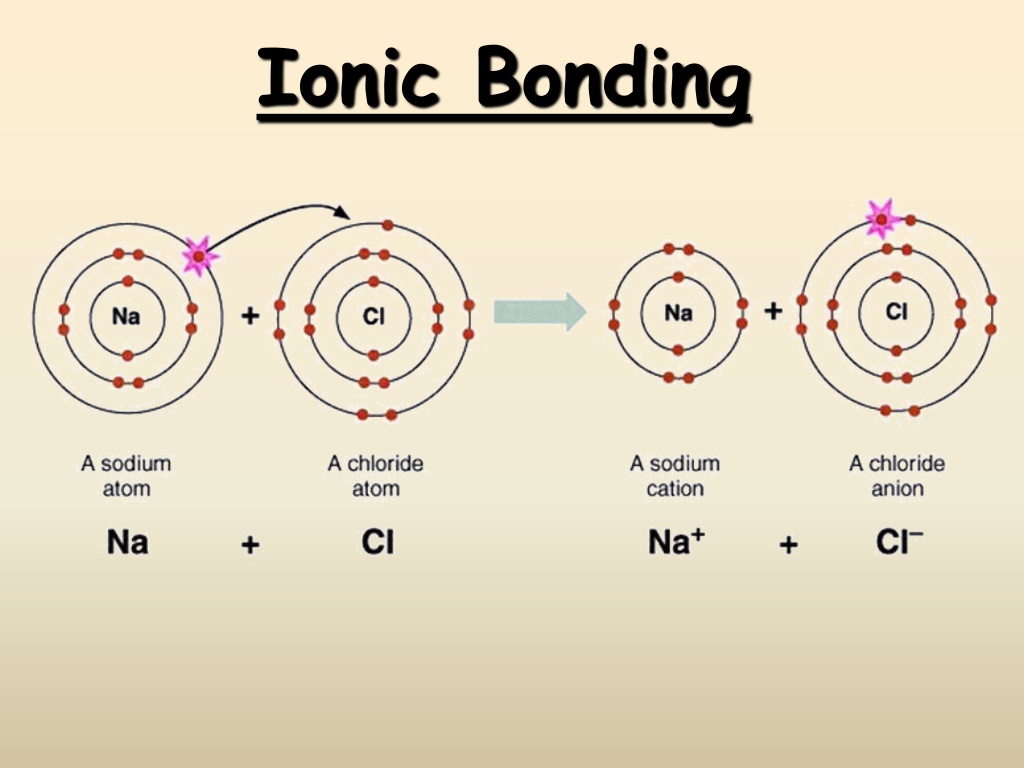
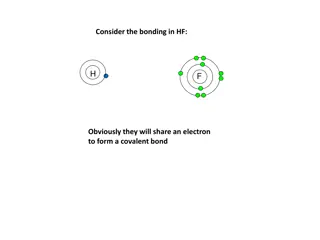
Ionic Bonding: The Formation of Sodium Chloride Sodium has 1 valence electron Chlorine has 7 valence electrons An electron transferred gives each an octet Na: 1s22s22p63s1 Cl: 1s22s22p63s23p5
Ionic Bonding: The Formation of Sodium Chloride This transfer forms ions, each with an octet: Na+ 1s22s22p6 Cl- 1s22s22p63s23p6
Ionic Bonding: The Formation of Sodium Chloride The resulting ions come together due to electrostatic attraction (opposites attract): Na+ Cl- The net charge on the compound must equal zero
Examples of Ionic compounds Mg2+Cl-2 Magnesium chloride: Magnesium loses two electrons and each chlorine gains one electron Na+2O2- Sodium oxide: Each sodium loses one electron and the oxygen gains two electrons Al3+2S2-3 Aluminum sulfide: Each aluminum loses two electrons (six total) and each sulfur gains two electrons (six total)
Metal Monatomic Cations Li+ Na+ K+ Mg2+ Ca2+ Ba2+ Al3+ Ion name Lithium Sodium Potassium Magnesium Calcium Barium Aluminum Lithium Sodium Potassium Magnesium Calcium Barium Aluminum
Nonmetal Monatomic Anions F- Cl- Br- I- O2- S2- N3- P3- Ion Name Fluorine Chlorine Bromine Iodine Oxygen Sulfur Nitrogen Phosphorus Fluoride Chloride Bromide Iodide Oxide Sulfide Nitride Phosphide
Sodium Chloride Crystal Lattice Ionic compounds form solid crystals at ordinary temperatures. Ionic compounds organize in a characteristic crystal lattice of alternating positive and negative ions. All salts are ionic compounds and form crystals.
Properties of Ionic Compounds Structure: Melting point: Boiling Point: Electrical Conductivity: Solubility in water: Crystalline solids Generally high Generally high Excellent conductors, molten and aqueous Generally soluble