Research at Hughes Spalding. Updated Review and Approval Process
Navigate the research review and approval process efficiently as a busy clinician investigator at Hughes Spalding with insights on key responsibilities, approval levels, and contact information of relevant personnel. From pre-award tasks like facilitating reviews and gaining approvals to post-award
3 views • 6 slides
Title IX Training
Title IX regulations mandate that educational institutions provide training to personnel involved in addressing sexual harassment and discrimination. This training covers defining sexual harassment, conducting investigations impartially, avoiding bias, and ensuring fair treatment in grievance proces
0 views • 125 slides
HER2 Tyrosine Kinase Inhibitor Zongertinib Phase Ia/b Trial in Solid Tumors
Study on Zongertinib, a HER2 tyrosine kinase inhibitor, in patients with HER2 aberration-positive solid tumors. The trial involves multiple centers worldwide and aims to evaluate the efficacy of Zongertinib in treating various cancers harboring HER2 mutations. Financial disclosures of lead investiga
1 views • 13 slides
Huntington's Disease Research Opportunities with Dr. Jamie Herron
Dr. Jamie Herron, a Consultant Psychiatrist specializing in Huntington's Disease, presents research opportunities including sub-investigator roles in clinical trials like Wave SNP3 and biomarker studies. The portfolio outlines the genetic basis of Huntington's, associated symptoms, and the chance to
2 views • 6 slides
Transitioning to Leadership Roles in Research: Challenges and Responsibilities
Explore the journey of transitioning into leadership roles in research, uncovering different leadership styles, tips for success, and the responsibilities and challenges that come with becoming a Principal Investigator. Learn how to navigate the transition, inspire your team, and effectively manage
0 views • 19 slides
Embracing Team Science in Research: A Comprehensive Guide
Explore the transition from investigator-driven to large-team research, understanding the essence of team science, its benefits, challenges, and the stages of team development. Learn the critical aspects of forming, storming, and norming teams before writing a grant, emphasizing shared vision, confl
0 views • 13 slides
Cybersecurity Expert Shiva V. Parasram - Profile and Advice
Shiva V. Parasram is a renowned figure in cybersecurity, serving as a Director, Cybersecurity Instructor, Penetration Tester, and Forensic Investigator. He shares valuable insights, tools, and tips for handling security breaches and hacks effectively, along with essential advice for safeguarding you
0 views • 17 slides
Provider Training Programs for Event Management Alignment
This collection of training programs focuses on event management alignment for executive, management staff, and direct care professionals in the service provider industry. The programs cover responsibilities, protocols, and processes related to reportable event management in various service provider
2 views • 7 slides
Villanova IRB New Investigator Training Overview
Human Subject Research Protections at Villanova IRB, including regulatory background, review process, and key information for investigators. Learn about the Institutional Review Board structure, ethical principles, and historical background of human subjects research at Villanova University.
0 views • 62 slides
Understanding Regulatory Requirements of Drugs and Pharmaceuticals
Drug regulation involves controlling drug use through international agreement authorities like the FDA, EMA, and PMDA. The FDA plays a crucial role in drug evaluation and research, biologic evaluation, devices, and food safety. There are various types of applications for drug approval, along with a
0 views • 28 slides
Understanding IRB Reliance Agreements in Research
IRB reliance agreements allow institutions to delegate IRB review responsibilities or collaborate with other institutions for human subjects research. Federalwide assurance (FWA) documents ensure compliance with regulations. Different types of agreements, such as IRB authorization agreements and ind
0 views • 23 slides
POSNOC & ATNEC Trials Update by Amit Goyal: Investigating Axillary Treatment in Breast Cancer
POSNOC (Positive Sentinel Node) and ATNEC trials are investigating the effectiveness of axillary treatment in women with early-stage breast cancer having metastases in one or two sentinel nodes. Led by Chief Investigator Amit Goyal in the UK, this randomized controlled trial compares adjuvant therap
1 views • 21 slides
Understanding the Notice of Award in Research Grants Management
The Notice of Award (NoA) is a crucial document in the grants management process that legally binds the grantee and establishes funding levels and conditions. It includes key information such as the grantee's details, principal investigator, funding period, and contact information. The NoA has stand
0 views • 49 slides
Transition to Leading a Research Team: A Guide for Emerging Leaders
Explore the journey of transitioning into a leadership role within a research team, covering different leadership styles, challenges, responsibilities, and tips for success. Gain insights on what to expect as you progress to becoming a Principal Investigator, managing funding, research advancement,
0 views • 19 slides
Comparison of FFR-guided PCI vs Angiography-guided PCI in AMI with Multivessel Disease: FRAME-AMI Trial
In patients with acute myocardial infarction (AMI) and multivessel coronary artery disease, this study aims to compare fractional flow reserve (FFR)-guided PCI with angiography-guided PCI for non-infarct-related artery lesions. The hypothesis is that selective PCI guided by FFR is superior to routin
2 views • 23 slides
Ensuring Protocol Compliance and Corrective Action Plans in Clinical Trials
This content discusses the importance of creating Corrective Action and Preventive Action (CAPA) plans for protocol deviations in clinical trials. It covers the components of a CAPA, best practices for creating CAPAs for different deviation types, and regulatory compliance requirements according to
0 views • 33 slides
REDCap Cloud: Advancing Clinical Trials with Enhanced Features
Streamline your clinical trial processes with REDCap Cloud, a secure and validated platform offering improved user interface, pre-configured ePRO, and compliance with regulations. Benefit from free licensing for investigator-initiated trials and explore the easy setup steps for creating forms and ev
0 views • 11 slides
Investigator Roles and Responsibilities in Clinical Research
Key responsibilities of investigators in clinical research involve ensuring participant safety, compliance with regulations, accurate data collection, and ethical conduct throughout the study process. Properly trained investigators play a crucial role in the success and integrity of clinical trials.
2 views • 81 slides
Principal Investigator Responsibilities in Clinical Trials
The Principal Investigator (PI) plays a crucial role in conducting clinical trials. Responsibilities include overseeing the trial at the site, making critical decisions, ensuring compliance with protocols, obtaining informed consent, maintaining accurate records, and more. Non-compliance can lead to
0 views • 36 slides
Data Editing and Coding Processes in Research
Data editing involves the detection and correction of errors in raw data to ensure accuracy and consistency. It includes field editing, where investigator reviews data for completeness, and central editing for thorough editing on completed forms. The coding process assigns symbols to responses for a
0 views • 7 slides
Best Practices for Subrecipient Monitoring in Research Projects
Effective management of subawards in research projects requires collaboration, clear responsibilities, financial monitoring, and technical oversight. This involves building strong relationships with stakeholders, including the Principal Investigator and subawardee, and ensuring compliance with spons
0 views • 10 slides
Embedded Research Conference Workgroup B: Management Decisions Support
Workgroup B at the Embedded Research Conference focuses on providing research support for management decisions. The participants aim to identify and prioritize operational questions that could benefit from embedded research, work productively within the existing QI ecosystem, and create an inventory
0 views • 14 slides
Study on Juvenile Drug Crime Prevention in Taiwan: Insights from ACS Conference 2020
This presentation at the Asian Criminological Society Conference 2020 delves into the current situation, dilemmas, and responses regarding juvenile drug crime prevention in Taiwan. The presenter, Zeng Li-Wen, a PhD student at Central Police University in Taiwan, provides valuable insights based on t
2 views • 20 slides
Rural Access Compliance Rules Proposal by Glenn Disher - PBM Investigator
Proposal by Glenn Disher, a PBM Compliance Investigator, outlines rules for rural access compliance. The proposal focuses on considering local conditions and enforcing rules for maximum impact. It includes recommendations for zip code rules, compliance mileage rules, and examples of non-compliant ru
0 views • 7 slides
Guide to Material Transfer Agreements and Data Negotiations in Research
Explore the key aspects of Material Transfer Agreements (MTAs), Confidentiality Agreements, and Data Use Agreements in research projects. Learn about the types of agreements negotiated, entities involved, the process for obtaining fully executed agreements, and how to get started as a Principal Inve
2 views • 28 slides
Research Ethics in Child Psychiatry: Introduction and Historical Perspectives
This presentation provides an introduction to research ethics in child psychiatry, emphasizing the importance of ethical considerations in conducting studies involving children. It highlights key ethical principles, historical events shaping research ethics, and examples of past unethical practices.
1 views • 39 slides
GMAS Proposal Entry and Homepage Enhancements Overview
GMAS Proposal Entry and Proposal Homepage are undergoing enhancements, consolidating multiple screens into one for Initial Proposal and Competing Renewal entries. Changes include smarter organization look-up, streamlined principal investigator search, new sponsor look-up, and automatic period genera
0 views • 17 slides
Comprehensive Overview of myResearch Portal for Sponsored Research at ORIS
Real-time web-based access to administrative data related to primary investigator's sponsored research at ORIS. The portal includes features for compliance approvals, financial accounts management, and delegation control. Access is granted to PSU employees with DUO authentication. Financial data fro
0 views • 18 slides
Sponsor Expectations for Clinical Studies at Thomas Jefferson University
This presentation discusses the sponsor expectations for clinical studies, investigator responsibilities, TJU's strengths in conducting studies, and how principal investigators can impress sponsors. Topics include assessment of investigator performance, regulatory guidelines, streamlining processes,
0 views • 11 slides
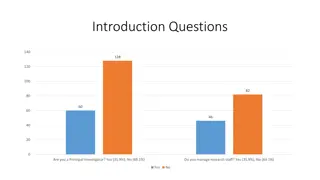
Survey Results on Research Training and Educational Support in Clinical Research Settings
This presentation showcases survey data on various aspects of research training and educational support in clinical research environments, including questions on being a Principal Investigator, managing research staff, training adequacy, study types, tenure at the University of Chicago, training pre
0 views • 35 slides
Two-Year Outcomes with Fully Repositionable Aortic Valve in High Surgical Risk Patients
The REPRISE II trial extended cohort evaluated the Lotus Valve System's safety and performance in symptomatic patients with severe aortic stenosis at high risk for surgical valve replacement. This prospective, single-arm study assessed the valve system's effectiveness through various follow-ups and
0 views • 19 slides
Initiating Sponsored Projects: A Comprehensive Guide
Explore the process of starting sponsored projects including the role of the Principal Investigator, required forms for personnel, proposal development steps, and review considerations before submission. Understand key aspects such as financial conflict of interest policies, intellectual property ag
0 views • 14 slides
Hire the Best Private Investigator in Malibu at Kinsey Investigations
As the leading private investigator in Malibu, Kinsey Investigations offers a wide array of services tailored to your needs. Our experienced team is adept at handling sensitive cases, ensuring confidentiality and professionalism at every step. From p
0 views • 6 slides
Hire the Best Private Investigator in Malibu
As the leading private investigator in Malibu, Kinsey Investigations offers a wide array of services tailored to your needs. Our experienced team is adept at handling sensitive cases, ensuring confidentiality and professionalism at every step. From p
0 views • 6 slides
Choose The Best Private Investigator in Malibu
As the leading private investigator in Malibu, Kinsey Investigations offers a wide array of services tailored to your needs. Our experienced team is adept at handling sensitive cases, ensuring confidentiality and professionalism at every step. From p
0 views • 6 slides
MUSC College of Medicine Promotion and Tenure Process
The MUSC College of Medicine outlines the detailed process and criteria for appointments, promotions, and tenure. It involves submission of packets to committees, reviews by subcommittees and full committees, and final recommendations for approval or disapproval. Specific criteria are provided for f
0 views • 28 slides
Guidelines for Academic Promotions in Medical School
Academic promotions in a medical school are crucial for recognizing achievements, maintaining competitiveness, and serving the institution's interests. Promotion criteria include teaching effectiveness, scholarly activity, clinical service, and active participation in various communities. Meritoriou
0 views • 47 slides
Private Investigator in Malibu
Seeking a reliable Private Investigator in Malibu? Kinsey Investigations specializes in discreet and effective solutions for all your investigative needs. Contact us today for professional assistance you can trust.
0 views • 6 slides
Understanding Pharmacology and Toxicology in Investigator Brochures
Explore the essential aspects of pharmacology and toxicology covered in Investigator Brochures, including nonclinical information, safety pharmacology, general toxicology, genetic toxicology, and more. Learn about the significance of pharmacology in predicting intended and unintended effects, consid
1 views • 40 slides
Challenges and Concerns in Immunology Research Funding: Investigator's Perspective
Investigator's face challenges in understanding NIH funding priorities, decreases in basic science grants, disease-earmarked funding changes, application logistics confusion, grant funding mechanisms variations, new rigorous research requirements, and equity in grant budgets.
0 views • 17 slides