Introduction to HEC-SSP
Explore the evolution and development of HEC-SSP, a software tool by the US Army Corps of Engineers, from its historical origins in the 1970s to its recent updates in analytical capabilities and user experience enhancements. Learn about the key team members behind the development and the new tools i
1 views • 52 slides
Hydrologic Modeling Methods in HEC-HMS: A Comprehensive Overview
Explore the transformative methods within HEC-HMS hydrologic modeling, including unit hydrograph derivation, excess precipitation transformation, hydrograph illustration, surface transform methods, and concepts like the kinematic wave and 2D diffusion wave. Learn about the unit hydrograph, kinematic
0 views • 41 slides
Introduction to Flood Risk Assessment with HEC-FDA Overview
This presentation delves into flood risk assessment using HEC-FDA software, covering topics such as defining flood risk, components of uncertainty, consequences of flood risk, and methods to assess flood risk including hydrology, hydraulics, geotechnical, and economics. It explores the intersection
6 views • 39 slides
Understanding Routing Methods in Hydrologic Engineering Center (HEC-ResSim)
Explore the differences between hydrologic and hydraulic routing, learn about open channel flow processes, and delve into channel routing within HEC-ResSim. Discover various reach routing methods, parameter estimation techniques, and calibration approaches. Dive into the Muskingum method and its app
6 views • 29 slides
Evolution of HEC-SSP Analytical Tools and Software Development Team
HEC-SSP, a software developed by the US Army Corps of Engineers, has evolved over the years to meet the statistical needs of the Corps. From its inception in FY2005 to the latest version, the software has seen significant enhancements in capabilities such as General Frequency, Curve Combination anal
9 views • 52 slides
Understanding Different Types of Scripts in HEC-ResSim
Explore the two main categories of scripts in HEC-ResSim - scripts executed outside simulations and scripts executed during simulations. Learn about the functions and properties of each type, including scripted rules and state variable scripts. Discover how these scripts compute flow limits, store m
6 views • 23 slides
Managing Water Resources Data with HEC-DSSVue
Learn about managing gridded data in HEC-DSSVue, including how grids represent spatial data, storing data in DSS, naming conventions, tools for manipulation, and more. Explore the importance of grids in hydrologic modeling and the application of spatial data in HEC-HMS and HEC-RAS.
4 views • 42 slides
Stochastic Storm Transposition in HEC-HMS: Modern Techniques and Applications
Explore the innovative methods and practical applications of Stochastic Storm Transposition (SST) in the context of HEC-HMS. Delve into the history, fundamentals, simulation procedures, and benefits of using SST for watershed-averaged precipitation frequency analysis. Learn about the non-parametric
3 views • 41 slides
GIS Data Management in Hydrologic Engineering Center (HEC) Software
Efficiently manage GIS data in HEC software by organizing background shapefiles, saving files in relevant directories, and utilizing relative pathnames. Understand the contents of basin files, GIS directories, and the step-by-step process involved in the delineation of elements within a project.
9 views • 5 slides
Overview of HEC API Scripting Part 1 and 2: DSS Files, DataContainer, HecTime, HecMath
This report delves into the HEC API scripting involving DSS files, DataContainer, HecTime, and HecMath. It covers important classes, accessing files, and the distinction between DataContainers and HecMath objects. Additionally, it compares Python and Java, highlighting their differences in interpret
2 views • 28 slides
FDA's Final Rule on Laboratory-Developed Tests (LDTs) and Phase-Out Policy
The FDA announced its Final Rule on laboratory-developed tests (LDTs), considering them as regulated medical devices. The phased-out policy will gradually enforce compliance with premarket review, quality system regulation, and other requirements over several stages. Additionally, a limited enforcem
2 views • 6 slides
FDA Perspective on Epidemiological Cut-off Values (ECVs)
The FDA presents insights on the development and use of Epidemiological Cut-off Values (ECVs) to distinguish wild-type populations from those with acquired resistance mechanisms. ECVs are crucial for determining antimicrobial susceptibility and guiding treatment decisions. The process involves analy
0 views • 13 slides
Experience in FDA Complex Innovative Designs Pilot Meeting: Incorporating External Controls in Phase 3 Study for DLBCL
Explore the experience of participating in the FDA's CID Pilot Meeting, focusing on designing a Phase 3 study for DLBCL with an external control arm for secondary overall survival. Discover key considerations for future studies with external controls to address high unmet medical needs in DLBCL pati
0 views • 13 slides
Understanding FDA Regulations and Medical Device Classification
The Food and Drug Administration (FDA) plays a crucial role in regulating research, manufacturing, marketing, and distribution of medical devices. Medical devices are classified based on risk and intended use, with three main categories determining regulatory pathways. The classification system help
2 views • 27 slides
Hydrologic Modeling with Gridded Precipitation Data
Learn about utilizing gridded precipitation data for hydrologic modeling with HEC-HMS. Explore the advantages of spatially distributed precipitation sources such as RADAR and gauge comparisons, and understand the various national and regional products available. Discover utilities for converting and
3 views • 14 slides
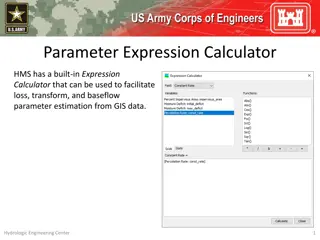
Parameter Expression Calculator for Efficient Parameter Estimation from GIS Data
Parameter Expression Calculator within HEC-HMS offers a convenient tool to estimate loss, transform, and baseflow parameters using GIS data. It includes various options such as Deficit and Constant Loss, Green and Ampt Transform, Mod Clark Transform, Clark Transform, S-Graph, and Linear Reservoir. U
2 views • 5 slides
Restructuring of USACE HEC-HMS Meteorologic Model
Significant modifications have been made to the HEC-HMS meteorologic model to enhance modeling tasks' ease and intuitiveness. The Met Model Restructure updates in versions 4.9 to 4.11 streamline meteorologic processes, introduce new features like automatic linkages and zonal editors for snowmelt, an
0 views • 19 slides
Historic Precipitation Hydrologic Modeling with HEC-HMS Slides by Greg Karlovits, Emily Moe, Daniel Black
Delve into the world of hydrologic modeling with HEC-HMS through a detailed presentation by experts Greg Karlovits, Emily Moe, and Daniel Black. Explore meteorologic models, atmospheric boundary conditions, basin modeling, and simulation runs in this informative slide series.
0 views • 12 slides
Understanding the FDA Audit Process in Research Compliance
The FDA conducts audits to ensure compliance with regulations in research settings. The process involves site notification, preparation, visit, and final determinations. Site preparation includes alerting staff, reserving rooms, ensuring access to necessary equipment, and preparing relevant document
0 views • 17 slides
Understanding Regulatory Requirements of Drugs and Pharmaceuticals
Drug regulation involves controlling drug use through international agreement authorities like the FDA, EMA, and PMDA. The FDA plays a crucial role in drug evaluation and research, biologic evaluation, devices, and food safety. There are various types of applications for drug approval, along with a
0 views • 28 slides
Understanding the NDA and ANDA Regulatory Approval Process
The New Drug Application (NDA) submission process to the FDA involves extensive documentation of non-clinical, clinical, and drug chemistry data to support the product's labeling. Key decisions during the FDA review include evaluating the drug's safety, effectiveness, labeling, manufacturing methods
0 views • 19 slides
Understanding Canopy and Surface Methods in HEC-HMS
Explore the various canopy and surface methods utilized in HEC-HMS for managing water resources. Learn about canopy interception, evapotranspiration, common parameter values, and factors affecting losses. Delve into available methods, canopy storage values, and surface depression storage. Enhance yo
0 views • 12 slides
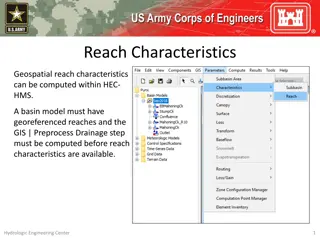
Understanding Reach Characteristics in HEC-HMS Basin Modeling
Reach characteristics play a crucial role in hydrological modeling using HEC-HMS. Georeferenced reaches are essential for computing reach characteristics like slope, sinuosity, relief, and more. The process involves pre-processing drainage data before detailed reach characteristics can be obtained.
0 views • 5 slides
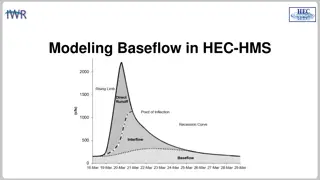
Understanding Baseflow Modeling in HEC-HMS
Baseflow in HEC-HMS plays a crucial role in modeling runoff volume, consisting of groundwater flow components like interflow and baseflow. Different approaches are used to model baseflow, with HEC-HMS currently incorporating conceptual methods. The response times for interflow and baseflow differ, i
1 views • 7 slides
Understanding Scripting in HEC-ResSim
Explore the two categories of scripts in HEC-ResSim - executed outside simulations and during simulations. Learn about static scripts for pre-processing, running simulations, and post-processing, along with the tools like Script Selector and Script Editor for script execution and editing.
0 views • 34 slides
Ethical Protections in Research: Historical Perspectives and Training Objectives
Explore the evolution of ethical protections in research involving human subjects, from key historical events to current regulations governing VA research. Understand the responsibilities of investigators and entities in the approval process, along with pathways for accessing drugs via FDA's Expande
0 views • 25 slides
HEC-ResSim Alternatives and Simulations Overview
Explore the functionalities of HEC-ResSim including creating, composing, and setting up alternatives for hydrological engineering simulations. Learn about ResSim alternatives, simulation modules, and the alternative editor in detail.
0 views • 31 slides
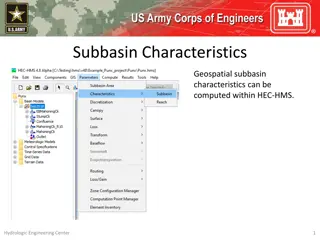
Understanding Subbasin Characteristics in HEC-HMS
Subbasin characteristics play a crucial role in hydrological modeling within HEC-HMS. Before computing these characteristics, the basin model must have georeferenced subbasins in the GIS. Various parameters such as basin slope, relief, elongation ratio, drainage density, and flowpath lengths and slo
0 views • 13 slides
Understanding Investigational New Drug Applications (INDA)
An Investigational New Drug Application (INDA) is a crucial submission to the FDA for permission to conduct clinical studies on new drug products. It plays a pivotal role in assessing the safety and efficacy of new drugs before they can be marketed and distributed for human use. This article covers
0 views • 30 slides
Targeted Learning Framework for Causal Effect Estimation Using Real World Data
Hana Lee, Ph.D., presents a webinar on the Targeted Learning Framework for Causal Effect Estimation using Real World Data (TMLE). The project aims to help the FDA develop a structured approach to incorporating real-world data into regulatory decision-making. TMLE offers a systematic roadmap aligned
0 views • 27 slides
Understanding the FDA Approval Process for Medical Devices
The FDA approval process for new medical devices involves rigorous evaluations to ensure safety and effectiveness. Conflicting criticisms of the FDA focus on the balance between tightening or loosening regulations without compromising public health. The agency's mission emphasizes protection and adv
0 views • 19 slides
fda registration
IAS helps organizations to register their products in US FDA. It is always a tedious process to collect and file the application as the US-FDA has got stringent rules. We are having experience in US-FDA registration thus we put forward our Services t
3 views • 2 slides
Comprehensive Human Subjects Protection Training for VA Personnel
This training addresses the ethical principles and regulations governing VA research involving human subjects, focusing on conducting FDA-regulated Expanded Access Program activities for Monkeypox treatment. It covers historical events shaping research practices, key responsibilities of investigator
0 views • 25 slides
Examples of Real-World Evidence in Regulatory Decisions by FDA
FDA showcases illustrative examples of how Real-World Evidence (RWE) has been utilized in regulatory decisions, highlighting its value across different submission types, data sources, and purposes from FY12-FY19. The FDA report features cases like a modified hemodialysis catheter end cap, tumor prof
0 views • 14 slides
Unregulated Risks of E-Cigarettes: FDA and Safety Concerns
E-cigarettes, unregulated and not FDA-approved, pose health risks with potential carcinogens and toxic chemicals. Lack of oversight allows for manufacturers to sell products with varying safety standards. FDA testing has revealed concerning findings, yet regulations are limited in addressing key iss
0 views • 40 slides
Expert Tips for Successful FDA Inspections
Gain insights from Joyce Nancarrow Tull, an experienced professional, on navigating FDA inspections successfully. Learn about legal authority, inspection procedures, key strategies, and more. Be proactive, seek expert advice, and build knowledge to enhance your readiness for FDA audits.
0 views • 31 slides
Understanding the Structure and Functions of FDA in the USA and Canada
The Food and Drug Administration (FDA) in the USA is a critical agency within the Department of Health and Human Services responsible for regulating the safety of various products such as foods, drugs, medical devices, and cosmetics. The FDA has distinct organizational units like the Office of the C
0 views • 49 slides
Understanding FDA Regulations in Clinical Research
FDA regulations in clinical research cover the protection of human subjects, IND/IDE requirements, risk determinations, application processes, and what falls under FDA regulation. It distinguishes between FDA-regulated activities and those not regulated, such as medical record reviews. The scope inc
0 views • 31 slides
Bioanalysis Validation Case with Michael Cohen-Wolkowiez at Duke University
Michael Cohen-Wolkowiez, MD PhD, a Pediatric Trials Network Professor at Duke University, conducted a bioanalysis validation case study involving Meropenem in infants funded by NIH. The study focused on PK and safety data for FDA registration consideration. High-level results showed significant diff
0 views • 6 slides
Off-Label Marketing and Free Speech in the Post Caronia and Amarin World
Background of off-label use and prohibition, First Amendment challenges to FDA authority, next steps for industry and FDA in responding to the new legal landscape. FD&C Act prohibits misleading labeling, FDA's regulatory authority on drug and device promotions, FDA's view on off-label promotion proh
0 views • 19 slides