Types of groups and reactions
This information discusses electron-donating groups (EDGs) and electron-withdrawing groups (EWGs), their effects on molecule reactivity, examples of each group, nucleophiles, and electrophiles. EDGs increase electron density, making nucleophiles stronger, while EWGs decrease electron density, making
0 views • 14 slides
Aromatic compounds
Aromatic compounds are chemical compounds containing a ring with delocalized pi electrons. Learn about the definition, naming conventions, and reactions of aromatic compounds such as benzene. Explore topics like aryl groups, halogenation reactions, and the mechanism for bromination of benzene.
0 views • 19 slides
Understanding Lewis Symbols in Chemistry
Lewis symbols are used to describe electron configurations in atoms and molecules. They help in understanding how elements form bonds by sharing or transferring electrons. The Octet Rule guides the formation of compounds, but exceptions exist. Double and triple bonds show atoms sharing multiple elec
0 views • 10 slides
Understanding Models of the Atom: Nucleus, Protons, Electrons
Dive into the fundamental concepts of atoms, exploring their structure with a focus on the nucleus, protons, electrons, and neutrons. Discover how the periodic table reveals crucial information about elements, such as atomic number and mass number. Explore the evolution of atom models and the necess
3 views • 20 slides
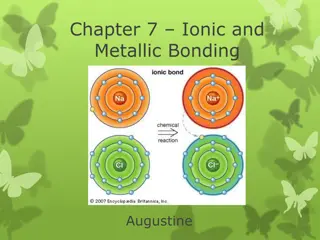
Understanding Ionic and Metallic Bonding: Valence Electrons, Octet Rule, and Ion Formation
Explore the essential concepts of ionic and metallic bonding, focusing on valence electrons, electron dot structures, the octet rule, cations, anions, and ion formation. Discover how atoms achieve stability through electron transfer, and learn to write electron configurations for various ions.
9 views • 52 slides
Understanding Ionic and Metallic Bonding in Chemistry
Explore the concepts of ionic and metallic bonding in chemistry through discussions on valence electrons, electron dot structures, the octet rule, cations, anions, and more. Dive into the world of ions and electron configurations to understand how atoms achieve stability through the gain or loss of
3 views • 62 slides
Understanding the Periodic Table and Chemical Bonds in Physical Science
The periodic table organizes elements based on their properties, with rows representing periods and columns representing groups. Mendeleev's early table laid the foundation for predicting undiscovered elements. Today's periodic table orders elements by atomic number, showcasing the periodic law and
3 views • 15 slides
Understanding Electrons in Atoms: Models and Quantum Mechanics
Explore the evolution of atom models from Rutherford to Bohr and the Quantum Mechanical Model. Learn about energy levels, electron movement, excitations, and orbital probabilities. Discover how electrons jump between energy levels and the limitations of Bohr's model in multi-electron atoms.
1 views • 51 slides
Understanding Chemical Bonds and Ionic Compounds
Ionic bonds are formed when atoms transfer electrons to achieve stable electron configurations, resulting in the creation of ions with positive or negative charges. Metals are good conductors due to their ability to easily lose electrons. The charges of ions depend on the number of valence electrons
0 views • 49 slides
The Building Blocks of the Universe: Protons, Neutrons, Electrons, Atoms, and Beyond
The universe is composed of just seven different things - protons, neutrons, and electrons- the fundamental building blocks of matter. These particles combine to form atoms like hydrogen, helium, and carbon, which are essential components of our bodies. Understanding the structure and properties of
0 views • 37 slides
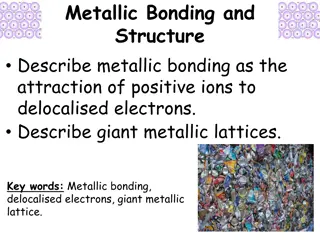
Understanding Metallic Bonding and Giant Metallic Lattices
Metallic bonding involves the attraction of positive metal ions to delocalized electrons, forming giant metallic lattices. In this structure, positive metal ions occupy fixed positions while electrons move freely throughout. This bonding is different from covalent bonding as it is delocalized, leadi
1 views • 19 slides
Understanding Electron Theory and the Structure of Matter
The structure of matter is intricately tied to electron theory, where molecules are made up of atoms containing protons and electrons. An electrically neutral atom has an equal number of protons and electrons. A flow of electrons creates an electric current, which can be measured by an ammeter. Cond
0 views • 11 slides
Chemistry Concepts: Valence Electrons, Ion Charges, and Ionic Compounds
Explore various key concepts in chemistry such as valence electrons in magnesium, Lewis Dot structure for silicon, charges on ions like strontium, formation of ions to achieve noble-gas electron configuration, elements forming ions with specific charges, and the octet rule. Learn about the character
1 views • 48 slides
Understanding the Impact of Temperature on Fermi Level in Semiconductors
The Fermi level plays a crucial role in determining the behavior of electrons in semiconductors at different temperatures. As temperature increases, the Fermi level shifts, affecting the generation of free electrons and holes in the valence and conduction bands. In intrinsic semiconductors, electron
2 views • 53 slides
Magnetic Force and Acceleration of Electrons in Television Picture Tubes
An electron in a television picture tube is analyzed as it moves towards the front of the tube in a magnetic field. The magnetic force and acceleration of the electron are calculated, along with determining the linear speed of a proton moving in a circular orbit under a magnetic field. Additionally,
0 views • 6 slides
Exploring the Nature of Subatomic Particles and Light
Explore the intricate world of subatomic particles such as electrons, protons, and neutrons, and delve into the dual nature of light as both particles and waves. Discover the structure of atoms, their isotopes, atomic number, mass number, and the fundamental discoveries in the field of physics, incl
1 views • 16 slides
G.P. Thomson's Experiment: Confirmation of Matter Wave Nature of Electrons
The experiment conducted by G.P. Thomson in 1928 confirmed the matter wave nature of electrons through diffraction patterns obtained when high-speed electrons were diffracted from a thin metallic film. The setup involved accelerating electrons through a high potential, incident on a gold foil, and t
0 views • 6 slides
Understanding Atomic Structure: Electrons, Energy Levels, and Historical Models
The atomic model describes how electrons occupy energy levels or shells in an atom. These energy levels have specific capacities for electrons. The electronic structure of an atom is represented by numbers indicating electron distribution. Over time, scientists have developed atomic models based on
0 views • 5 slides
Understanding Valence Electrons in Chemistry
Explore the concept of valence electrons with definitions, diagrams, and explanations of how electrons are located in the electron cloud. Learn about the importance of valence electrons in determining an element's reactivity and stability. Discover how atoms gain or lose electrons to achieve a full
0 views • 13 slides
Understanding Valence Electrons and Ionic Charges in Elemental Bonding
Valence electrons play a crucial role in the formation of ions as elements combine. Nonmetals gain electrons to become negatively charged ions, while metals lose electrons to become positively charged ions. This process leads to the creation of electrically attractive elements open for bonding. The
0 views • 17 slides
Understanding the Principle and Working of Semiconductor Lasers
Semiconductor lasers operate through absorption, spontaneous emission, and stimulated emission processes. Absorption of radiation causes electrons to jump to higher energy levels, leading to light emission. Spontaneous emission is when excited electrons return to ground state by emitting photons, wh
3 views • 17 slides
Understanding Atoms, Ions, and Isotopes in Chemistry
Atoms are neutral with equal protons and electrons. Ions are charged atoms resulting from gaining or losing electrons, while isotopes are atoms with varying numbers of neutrons. The atomic number always signifies the number of protons in an atom, unaffected by electron or neutron changes. Explore th
2 views • 5 slides
Understanding Valence Electrons in Atoms and the Periodic Table
Explore the concept of valence electrons in atoms, crucial for chemical bonding. Learn to define valence electrons, understand their role, and draw electron-dot structures for atoms. Practice identifying valence electrons in various elements and creating electron-dot structures in this educational c
1 views • 12 slides
Understanding Bonding in Chemistry
Delve into the world of chemical bonding through ionic, covalent, and metallic bonds. Explore how elements form bonds, from the attraction between sodium and chloride ions to the sharing of electrons in covalent bonds. Witness the formation of compounds like sodium chloride and magnesium oxide, unde
1 views • 12 slides
Understand Molecular Structures with Lewis Dot Symbols
Explore the world of molecular structures with Lewis dot symbols in this chemistry unit. Learn about valence electrons, covalent bonding, and the HONC 1234 rule through engaging activities and discussions. Create accurate structural formulas and describe bonding in molecular substances. Get ready to
0 views • 13 slides
Measuring the e/m Ratio Experiment: Understanding Electrons' Behavior in Magnetic Fields
In 1897, JJ Thomson proved electrons were small negatively charged particles through cathode ray experiments. Today, you will replicate a similar experiment to determine the charge-mass ratio of an electron. With the provided apparatus, understand the force on electrons in a magnetic field and obser
0 views • 23 slides
Understanding Periodic Trends: Atomic Radius and Ionization Energy
The Periodic Table displays a systematic organization of elements based on their increasing atomic number, leading to periodic patterns in their physical and chemical properties. Key trends like Atomic Radius and Ionization Energy provide insights into the size of atoms and the energy required to re
1 views • 13 slides
Understanding the 18-Electron Rule in Transition Metal Organometallic Compounds
The 18-electron rule governs the stability of transition metal organometallic compounds by requiring the sum of metal d electrons and ligand-supplied electrons to be 18. This rule highlights the importance of electron count and ligand characteristics in forming stable complexes. Key concepts include
0 views • 15 slides
Understanding Shielding and Slater's Rule in Chemistry
Explore the concept of shielding and Slater's rule in chemistry, which explains how electrons in different energy levels affect the effective nuclear charge experienced by valence electrons. Learn how to estimate shielding extent using Slater's rules and calculate the effective nuclear charge for el
0 views • 5 slides
Understanding the Periodic Table and Valence Electrons
Dmitri Mendeleev's organization of elements into the periodic table, the layout of periods and groups, and the significance of valence electrons in determining chemical reactivity are explored in this informative content. Discover how valence electrons relate to group numbers and learn about excepti
0 views • 31 slides
Runaway Electrons in Tokamak Disruptions: Current Status and Future Directions
The article discusses the current knowledge and open questions regarding runaway electrons in tokamak disruptions, focusing on prospects for ITER tools and plans. It covers theoretical frameworks, RE generation mechanisms, validation models, and challenges in disruption scenarios. The need for self-
0 views • 20 slides
Introduction to Drude Model in Solid State Physics
Drude Model, formulated around 1900, explains the fundamental properties of metals such as electricity and heat. It proposes that electrons in metals behave like a classical electron gas, moving freely between atomic cores. The model considers the mean free path between electron collisions and estim
0 views • 39 slides
Understanding Valence Electrons and Lewis Dot Diagrams
Explore the concept of valence electrons and Lewis dot diagrams in chemistry. Learn how to identify the number of protons, neutrons, and electrons in an element using Bohr model drawings. Discover the significance of valence electrons in bonding and how to determine the number of valence electrons f
0 views • 37 slides
Understanding Ions and Their Importance in Your Body
Neutral atoms have the same number of protons and electrons, with the charge of the nucleus always positive. The mass number is the sum of protons and neutrons. The number of neutrons can be calculated by subtracting the atomic number from the mass number. Ions are atoms with a positive or negative
0 views • 15 slides
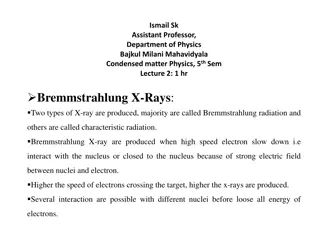
Understanding Bremmstrahlung and Characteristic X-Rays in Condensed Matter Physics
In condensed matter physics, Bremmstrahlung X-rays and characteristic X-rays are produced through different interactions of high-speed electrons with nuclei in target atoms. Bremmstrahlung radiation is generated when electrons slow down near the nucleus, while characteristic radiation is produced wh
0 views • 5 slides
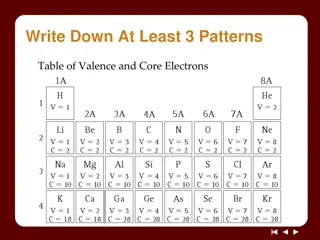
Understanding Electron Arrangements in Atoms
Explore the concepts of electron arrangements in atoms through activities and discussions on valence and core electrons, shell models, and patterns in the periodic table. Discover how the number of electrons in shells relates to element properties and learn to create shell model diagrams. Dive into
0 views • 14 slides
Understanding Atomic Orbitals: Counting, Subshells, Energies, and Electrons
Learn about the basics of atomic orbitals, including the counting of orbitals in shells and subshells, the distribution of electrons in different energy levels, and the symmetrical nature of orbital labeling. Dive into the rules governing electron placement based on quantum mechanics and explore the
0 views • 6 slides
Understanding Chemical Bonds and Molecular Geometry
Chemical bonds are the forces that hold atoms together, with valence electrons playing a crucial role. Ionic bonds involve complete electron transfer between metals and nonmetals, while covalent bonds see electrons being shared. Lewis dot diagrams help in visualizing the valence electrons of atoms,
0 views • 68 slides
Understanding Static Electricity and Electrostatics
Static electricity is a result of electric charge buildup on insulating materials due to friction, causing electrons to transfer and create a charge difference. This can lead to phenomena like a balloon sticking to a wall. The origin of static charge lies in the electrons and protons within atoms, w
0 views • 9 slides
Understanding Ionic Bonds: Valence Electrons and Ion Formation
Exploring the concepts of valence and core electrons, Noble Gas Envy Ions, and the formation of ions through electron transfer between metal and nonmetal atoms. Learn about cations and anions, ion charges based on the periodic table, and the relationship between ion charge and valence electrons.
0 views • 13 slides