Understanding the Periodic Table and Valence Electrons
Dmitri Mendeleev's organization of elements into the periodic table, the layout of periods and groups, and the significance of valence electrons in determining chemical reactivity are explored in this informative content. Discover how valence electrons relate to group numbers and learn about exceptions for groups 3 to 12 and elements in groups 13 to 18.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
DmitriMendeleev organized the known elements into a table called the periodic table. He organized them by their increasing atomic masses.
Today we organize the elements by their increasing Atomic Number.
Rows on the periodic table are called periods. All the elements listed in a row belong to the same period. There are 7 (seven) periods.
Columns in the periodic table are called groups or families. All elements in a group have similar properties.
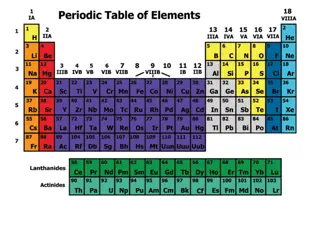
Insert copy of the periodic table. The columns have been given an Arabic number. The columns are numbered 1 18 starting at the left and moving to the right. Please make sure that your periodic table has them numbered like this.
Valence Electrons The outer most energy level is usually not full or complete . These electrons in the outer energy level are called Valence electrons. Valence electrons are important because they determine how an element will react with other substance.
Insert picture of periodic table. Group 1 and 2 the number of Valence electrons in the outer energy level will match the group number.
Insert picture of periodic table. Groups 3 through 12 do not follow any particular rule to determine the number of Valence electrons.
Insert picture of periodic table. Group 13 to 18 The Valence electrons for each of these is the group number less 10. For example Group 14 10 = 4 Valence electrons.
Elements whose atoms gain, lose or share electrons are reactive and they combine to form the many compounds we use in our daily lives.
Elements are categorized as metals, nonmetals or metalloids. Metals: An element that has luster, is malleable and ductile, and is said to be a good conductor of heat and electricity.
What is luster, malleable and ductile? Luster describes the way a surface reflects light therefore metallic luster would be shiny like a metal object. Malleable means to be able to press or pound the substance into sheets or different shapes. Ductile means that the substance can be drawn out into thin wires.
Elements are categorized as metals, nonmetals or metalloids (cont ). Nonmetals: an element that is usually a gas or a brittle solid at room temperature. It is a poor conductor of heat and electricity.
Elements are categorized as metals, nonmetals or metalloids (cont ). Metalloid: an element that shares some characteristics or properties with both metals and nonmetals.
Group 1 and 2 Elements: These elements are so reactive that they are only found combined with other elements in nature.
Group 1: Alkali Metals 3 Li Soft can be cut with a knife Lithium 6.941 11 Na Shiny and silver colored Sodium 22.990 Low Density (some will even float) 19 K Potassium 39.098 Most reactive of the metals 37 Rb Valence electrons = 1 Rubidium 85.468 Reacts violently with water forming a hydrogen gas 55 Cs Cesium 132.905 Compounds from these are very useful such as NaCl 87 Fr Francium 223.020
4 Group 2: Alkaline- Earth Metals Be Beryllium 9.012 Very reactive but not as reactive as Alkali Metals. 12 Mg Magnesium 24.305 Silver colored 20 Ca More dense than Group 1 metals Calcium 40.078 38 Sr Valence electrons = 2 Strontium 87.62 Useful compounds include: Calcium compounds such as cement, plaster, chalk, and YOU. 56 Ba barium 137.327 88 Ra Radium 226
Group 3 to 12: Transition Metals Do not lose their valence electrons as easily as groups 1 & 2. Less reactive than Alkali and Alkaline Earth metals Shiny Good conductors of electricity Higher density and melting points (except mercury) than Group 1 and 2
Group 3 to 12: Transition Metals (cont.) Lanthanides: The first row underneath the periodic table: Shiny, reactive, many are used in the production of steel. Actinides: The second row underneath the periodic table: These elements are all radioactive and unstable.. Note: Elements found after 94 (Plutonium) are man made and not found in nature
5 B Group 13: Boron Group Boron 10.811 13 Al Aluminum 26.982 31 Ga Gallium 69.723 49 In Indium 114.818 81 Tl Thallium 204.383 Reactive Valence electrons = 3 Contains 1Metalloid and 4 Metals Solid at room temperature Aluminum is the most abundant in this group and the most common in the Earth s crust
6 C Group 14: Carbon Group Carbon 12.011 14 Si Silicon 28.086 32 Ge Germanium 72.64 50 Sn Tin 118.710 82 Pb Lead 207.2 Reactivity varies in this group depending on the element Valence electrons = 4 This group contains 2 metals, 1 nonmetal and 2 metalloids. Many forms found uncombined in nature such as diamonds Compounds are very useful: proteins, fats, carbohydrates, computer chips.
Group 15: Nitrogen Group 7 N Reactivity varies in this group depending on the element Nitrogen 14.007 15 P Phosphorous 30.974 33 As Arsenic 74.922 51 Sb Antimony 121.760 83 Bi Bismuth 208.980 Valence electrons = 5 Group contains 1 metal, 2 Nonmetals, and 2 metalloids Phosphorous is very reactive and only found in nature combined with other elements. All but nitrogen are solid at room temperature. Nitrogen makes up 78% of our atmosphere. Generally unreactive.
Group 16: Oxygen Group 8 O More reactive than group 15 Oxygen 15.999 16 S Sulfur 32.065 34 Se Selenium 78.96 52 Te Tellurium 127.60 84 Po Polonium 209 Valence electrons = 6 Group contains 1 Metals, 3 Nonmetals and 1 Metalloids Sulfur is found in nature and is used to make sulfuric acid, a very commonly used chemical in industry. All but oxygen are solid at room temperature. Oxygen makes up 21%of the Earth s Atmosphere Oxygen is very reactive and combines with many other elements especially metals Rust is the result of the oxidation of metal.
Group 17: Halogen Group 9 F Very reactive Fluorine 18.998 17 Cl Chlorine 35.453 35 Br Bromine 79.904 53 I Iodine 126.904 85 At Astatine 210 Valence electrons = 7 Nonmetal group Poor Conductors of electricity and heat React violently with alkali metals to form salts Never found uncombined in nature Atoms of these elements only need to gain 1 electron to fill their outer shell Chlorine and Iodine are both in this group and can be combined to make disinfectants.
2 He Group 18: Noble Gas Group Helium 4.003 Non-reactant 10 Ne Neon 21.180 Valence electrons = 8 Outermost energy shell is full 18 Ar Colorless, odorless gases at room temp. Argon 39.948 Under normal conditions they do not react with other elements 36 Kr Krypton 83.80 All found on Earth in very small amounts 54 Xe Argon is the most common in the group Xenon 131.293 Their non-reactivity makes them very useful for light bulbs, helium for blimps and weather balloons. 86 Rn Radon 222
Hydrogen Stands Alone: 1 H Properties do not match the properties of any single group Hydrogen 1.008 Valence Electrons = 1 Easily looses that one valence electron Physical properties are like the nonmetal group Most abundant element in the Universe Its reactive nature makes it useful as a fuel for rockets.