Survey Results on Research Training and Educational Support in Clinical Research Settings
This presentation showcases survey data on various aspects of research training and educational support in clinical research environments, including questions on being a Principal Investigator, managing research staff, training adequacy, study types, tenure at the University of Chicago, training preferences, institutional support levels, and CITI Human Subject Protection awareness. Results offer insights into challenges and opportunities for improvement in supporting researchers and enhancing clinical research quality.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
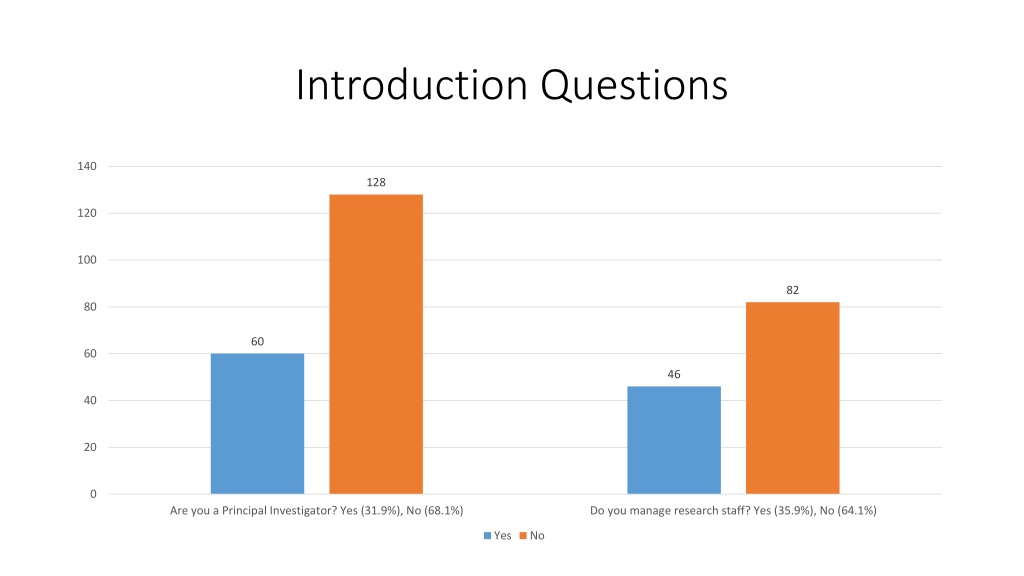
Introduction Questions 140 128 120 100 82 80 60 60 46 40 20 0 Are you a Principal Investigator? Yes (31.9%), No (68.1%) Do you manage research staff? Yes (35.9%), No (64.1%) Yes No
I have received sufficient training to succeed I have received sufficient training to succeed as a Principal Investigator. as a Principal Investigator. 25 21 20 16 15 10 5 3 2 1 0 Agree (44.7%) Disagree (34.0%) Strongly Disagree (6.4%) Unsure (4.3%) N/A (2.1%)
There is sufficient clinical research training for the staff who take on delegated responsibilities to succeed PI Response 18 17 16 15 14 12 10 8 7 6 4 4 2 2 1 0 Strongly agree (4.3%) Agree (32.6%) Disagree (37.0%) Strongly Disagree (8.7%) Unsure (15.2%) N/A (2.2%)
Describe your types of studies PI defined studies 35 30 30 25 20 15 10 7 7 4 5 0 Industry sponsored (14.6%) Investigator initiated (62.5%) Translation (14.6%) Other (8.3%)
How long have you (PI) been working at the University of Chicago? 30 25 20 15 10 5 0 Less than 1 year (2.1%) 1-3 years (10.4%) 3-10 years (37.5%) Over 10 years (50.0%) PI
Please indicate up to five training offering that you'd be interested in attending (PI) 18 16 16 16 14 13 13 14 11 12 10 10 9 9 10 8 8 7 8 5 5 6 4 4 3 3 3 4 2 1 2 0 0
The current level of institutional support meets your present clinical research educational needs. 30 26 25 20 PI 15 14 14 14 15 Manager 12 Non-PI/Manager 10 7 7 5 3 3 3 2 2 2 1 1 1 0 0 Strongly agree Agree Disagree Strongly Disagree Unsure N/A
CITI Human Subject CITI Human Subject Protection (required for IRB approval) (required for IRB approval) Protection 35 30 25 20 15 10 5 0 Unaware of this offering Aware of the offering, but not attended Have attended the training Have attended the training, and recommend it! N/A PI Manager NON (PI/Manager)
What institutional support do you need for your continued professional development? 40 36 34 35 32 29 30 26 23 23 25 22 21 21 19 18 20 16 15 15 11 10 10 7 4 3 5 1 1 0 Funds/sponsorship for educational training Mentoring Funds for professional certification(s) Resource list of available professional development options Time allocated per quarter/year for professional development Other N/A PI Manager NON PI/Manager
Good Clinical Practice (required by BSD for all human research) 35 32 29 30 25 20 15 15 13 12 9 10 5 4 4 5 2 1 1 1 1 0 0 Unaware of this offering Aware of the offering, but not attended Have attended the training Have attended the training, and recommend it! N/A PI Manager Non PI/Manager
OCR Fundamentals of Clinical Research 25 23 19 20 18 15 13 13 12 10 5 5 5 4 4 5 3 1 0 0 0 Unaware of this offering Aware of the offering, but not attended Have attended the training Have attended the training, and recommend it! N/A PI Manager Non PI/Manager
OCR Electives 30 25 25 20 18 15 13 13 13 10 10 8 7 7 4 5 1 1 1 0 0 0 Unaware of this offering Aware of the offering, but not attended Have attended the training Have attended the training, and recommend it! N/A PI Manager Non PI/Manager
OCR Town Halls / Workshops 18 17 17 16 16 16 16 15 14 12 10 8 6 5 5 4 4 3 3 3 2 2 0 0 0 Unaware of this offering Aware of the offering, but not attended Have attended the training Have attended the training, and recommend it! N/A PI Manager Non PI/Manager
Essentials of Patient-Oriented Research (EPOR) / RCR 40 35 30 25 20 15 10 5 0 Unaware of this offering Aware of the offering, but not attended Have attended the training Have attended the training, and recommend it! N/A PI Manager Non PI/Manager
CHeSS - Summer Program in Outcomes Research Training (SPORT) 50 45 40 35 30 25 20 15 10 5 0 Unaware of this offering Aware of the offering, but not attended Have attended the training Have attended the training, and recommend it! N/A PI Manager Non PI/Manager
CHeSS - Committee on Clinical and Translational Science (CCTS) academic courses 45 40 35 30 25 20 15 10 5 0 Unaware of this offering Aware of the offering, but not attended Have attended the training Have attended the training, and recommend it! N/A PI Manager Non PI/Manager
CHeSS - Quality Improvement and Patient Safety Course 45 40 35 30 25 20 15 10 5 0 Unaware of this offering Aware of the offering, but not attended Have attended the training Have attended the training, and recommend it! N/A PI Manager Non PI/Manager
CHeSS - Outcomes Research Workshop 45 40 35 30 25 20 15 10 5 0 Unaware of this offering Aware of the offering, but not attended Have attended the training Have attended the training, and recommend it! N/A PI Manager Non PI/Manager
ITM - Trio Chart Title 40 35 30 25 20 15 10 5 0 Unaware of this offering Aware of the offering, but not attended Have attended the training Have attended the training, and recommend it! N/A PI Manager Non PI/Manager
Graham School Master of Science in Biomedical Informatics 35 30 25 20 15 10 5 0 Unaware of this offering Aware of the offering, but not attended Have attended the training Have attended the training, and recommend it! N/A PI Manager Non PI/Manager
Graham School Certification Clinical Trial Management & Regulatory Compliance 30 25 20 15 10 5 0 Unaware of this offering Aware of the offering, but not attended Have attended the training Have attended the training, and recommend it! N/A PI Manager Non PI/Manager
Equatr 40 35 30 25 20 15 10 5 0 Unaware of this offering Aware of the offering, but not attended Have attended the training Have attended the training, and recommend it! N/A PI Manager Non PI/Manager
CRI Seminar Series (CRDW, REDCap, HPC) 40 35 30 25 20 15 10 5 0 Unaware of this offering Aware of the offering, but not attended Have attended the training Have attended the training, and recommend it! N/A PI Manager Non PI/Manager
URA Trainings 45 40 35 30 25 20 15 10 5 0 Unaware of this offering Aware of the offering, but not attended Have attended the training Have attended the training, and recommend it! N/A PI Manager Non PI/Manager
How long have you been working in clinical research? 30 25 20 15 10 5 0 Less than 1 year 1-3 years 3-10 years Over 10 years PI Manager Non PI/Manager
Do you have training in motivation, management of people, leadership acumen, managing remote employees? Manager 16 14 12 10 8 6 4 2 0 No (46.7%) Yes (13.3%) Yes, but interested in additional training (40.0%)
Preferred Training Methods Manager 12 10 8 6 4 2 0 Classroom lecture Virtual training - live Virtual training - recorded Hands-on training with an instructor Computer based learning - self-study Mentoring Shadowing First Choice Second Choice Third Choice
Preferred Training Methods Non PI/Manager 18 16 14 12 10 8 6 4 2 0 Classroom lecture Virtual training - live Virtual training - recorded Hands-on training with an instructor Computer based learning - self-study Mentoring Shadowing First Choice Second Choice Third Choice
Association of Clinical Research Professionals (ACRP) 20 18 16 14 12 10 8 6 4 2 0 Interested in training Interested in certification Not interested in training Not interested in certification Already Undecided or unsure N/A Member/Certified Manager Non PI/Manager
Society of Clinical Research Associates (SOCRA) 20 18 16 14 12 10 8 6 4 2 0 Interested in training Interested in certification Not interested in training Not interested in certification Already Undecided or unsure N/A Member/Certified Manager Non PI/Manager
Other Certifications (Select 5) Managers Evidence-based research skills (database search skills) (12, 40.0%), Regulatory Compliance for Clinical Research (10, 33.3%), Basic familiarity with resources availability at UCM (21, 70.0%), Technical Skills (Phlebotomy / Specimen Processing) (7, 23.3%), Presentation and poster creation (3, 10.0%), Publication skills (citation managers) (3, 10.0%), Management of multi-site studies (10, 33.3%), Clinical Research Budget / Contracting (7, 23.3%), Health-related apps for patients (4, 13.3%), Informed Consent Process (8, 26.7%), Protocol Development (13, 43.3%), IND/ IDE filings (7, 23.3%), Health-related apps for health professionals (2, 6.7%), Health literacy applications (2, 6.7%), IRB specific training (7, 23.3%), Data Safety Monitoring Boards (5, 16.7%), Statistical Plan Development (7, 23.3%), Use technology in research (REDCap, CRI, DW, EPIC) (14, 46.7%), Other (specify below) (1, 3.3%), N/A (0, 0.0%)
Other Certifications (Select 5) Non PI/Managers Evidence-based research skills (database search skills) (16, 31.4%), Regulatory Compliance for Clinical Research (20, 39.2%), Basic familiarity with resources availability at UCM (19, 37.3%), Technical Skills (Phlebotomy / Specimen Processing) (11, 21.6%), P resentation and poster creation (9, 17.6%), Publication skills (citation managers) (10, 19.6%), Management of multi-site studies (18, 35.3%), Clinical Research Budget / Contracting (20, 39.2%), Health-related apps for patients (7, 13.7%), Informed Consent Process (5, 9.8%), Protocol Development (14, 27.5%), IND/ IDE filings (9, 17.6%), Health-related apps for health professionals (4, 7.8%), Health literacy applications (7, 13.7%), IRB specific training (12, 23.5%), Data Safety Monitoring Boards (6, 11.8%), Statistical Plan Development (10, 19.6%), Use technology in research (REDCap, CRI, DW, EPIC) (14, 27.5%), Conducting research remotely (e-consent) (13, 25.5%), Other (specify below) (0, 0.0%), N/A (3, 5.9%)
The current level of institutional support meets your present clinical research needs for your staff as a Manager Series 1 14 12 10 8 6 4 2 0 Strongly agree ( 0.0%) Agree (20.0%) Disagree (43.3%) Strongly Disagree (13.3%) Unsure/Don't know (23.3%) N/A (0.0%) Series 1
What is your job title? 25 20 15 10 5 0 Manager Non PI/Manager
Please indicate which of the following describe your research position hours (Check all that apply) 45 40 40 35 30 27 25 20 16 15 9 10 8 8 8 7 7 6 5 5 0 0 Monday -Fridays 8-hour days Flexible weekdays, weekends and evenings Predominantly on campus Predominantly remote work Non PI/Manager Equal on campus/remote work hours Other Manager