India Alliance Clinical & Public Health fellowship in India
India Alliance Clinical & Public Health fellowship in India\n\nIndia Alliance Clinical and Public Health Research Fellowships are for Health researchers with an MD, MS, MPH, or an equivalent clinical or public health degree, who can apply for the DBT\/Wellcome Trust India Alliance Clinical and Publi
0 views • 5 slides
Training Models in Clinical Psychology: A Comprehensive Overview
Clinical psychology training models such as the Scientist-Practitioner Model have evolved over the years to integrate science and practical expertise. The Scientist-Practitioner Model, originating in 1949, emphasizes the fusion of scientific knowledge and clinical application. It faced criticism for
1 views • 98 slides
Ethical Issues in Clinical Pharmacy Research by Dr. Haider Raheem Mohammad
Research ethics play a crucial role in clinical trials and therapeutic research in the field of pharmacy. From discovery to validation, all medicines undergo rigorous evaluation processes to ensure safety, efficacy, and freedom from adverse effects. Clinical trials in both animals and humans are ess
0 views • 20 slides
Advanced Clinical Practice Framework and Pillars of Practice
The document discusses the advanced clinical practice framework and the four pillars of practice which include leadership & management, clinical practice, education, and research. It emphasizes the importance of core capabilities and area-specific competence in advanced clinical practice. The role o
2 views • 8 slides
Digital Health Technology-Derived Clinical Outcome Assessments in Regulatory Decision-Making
This session discusses the landscape of DHT-derived novel endpoints in clinical research, focusing on considerations for regulatory decision-making. It explains the use of digital health technology in clinical outcome assessments and highlights the potential benefits of digitally collected COAs, suc
0 views • 42 slides
Objective Structured Clinical Examination (OSCE): A Modern Approach to Assessing Clinical Competence
The Objective Structured Clinical Examination (OSCE) is a modern examination method widely used in the field of health science to evaluate clinical skill performance. It involves stations where medical students interact with simulated patients to demonstrate competencies such as history taking, phys
1 views • 40 slides
NIMH Clinical Research Education and Monitoring Program Overview
NIMH's Clinical Monitoring and Clinical Research Education, Support, and Training Program (CREST) aims to ensure the proper conduct, recording, and reporting of clinical trials. This program includes clinical monitoring plans, guidelines for site monitoring activities, and independent clinical monit
1 views • 29 slides
Understanding Statistical Methods for Clinical Endpoints in Diabetes Research
This educational slide module delves into fundamental statistics for analyzing clinical endpoints in diabetes research. It covers the choice of statistical methods, the distinction between statistical and clinical significance, and the importance of different endpoints in evaluating clinical benefit
1 views • 37 slides
Understanding Ethics in Clinical Trials: A Comprehensive Overview
Explore the historical context, important ethical guidelines, and the ethical framework with 7 principles in the field of clinical trials. Learn about key trials, ethical considerations, and guidelines governing human subject research in clinical medicine. Delve into the critical aspects such as inf
1 views • 15 slides
Understanding Non-Clinical Development in Therapeutic Innovation
The European Patients Academy on Therapeutic Innovation focuses on the non-clinical development phase of medicine, delving into efficacy assessment, safety evaluation, and manufacturing process considerations. Non-clinical studies are essential for decision-making in clinical trials, marketing appli
1 views • 26 slides
Overview of Society of Clinical Research Associates (SOCRA)
SOCRA is a non-profit organization dedicated to providing education, certification, and networking opportunities in clinical research. Established in 1991, it offers workshops, conferences, and a CCRP program for industry professionals. Attendees benefit from experienced presenters and opportunities
0 views • 18 slides
Epic Tools for Clinical Research by Shara Power, RN, BSN, OCN
Explore Epic tools for clinical research developed by Shara Power, a skilled application developer specializing in EPIC Beacon Oncology at UIHC Healthcare Information Systems. Learn about managing research study records, investigational study medication orders, and the process for creating and using
0 views • 26 slides
Epic Tools for Clinical Research by Shara Power, RN, BSN, OCN
Epic Tools for Clinical Research, developed by Shara Power, RN, BSN, OCN, offers a comprehensive suite of applications within the EPIC system for managing research study records, medication orders, treatment plans, billing, consent records, and patient associations. This solution streamlines the pro
0 views • 22 slides
BMC Clinical Trials Office Goals and Initiatives
The BMC Clinical Trials Office (CTO) is dedicated to supporting clinical and human research at Boston Medical Center by providing leadership and expertise in research, finance, and administration. The CTO reviews, negotiates, and approves protocols, supports grants and contracts, ensures accurate cl
0 views • 17 slides
Understanding Clinical Trials: Types and Designs
Clinical trials are essential research studies that evaluate new tests and treatments to improve human health outcomes. They involve various phases, designs, and purposes, such as treatment trials, prevention trials, and observational studies. Different types of clinical trial designs include experi
7 views • 18 slides
Enhancing Translational Research: Bridging the Gap Between Clinical and Basic Scientists
Translational research aims to translate basic scientific discoveries into practical applications for patient care. This involves moving research from the laboratory to clinical settings effectively and safely. Key aspects include designing studies using relevant models, documenting mechanisms of ac
0 views • 8 slides
Understanding Evidence-Based Medicine and Clinical Decision-Making
European Patients Academy on Therapeutic Innovation emphasizes the importance of Evidence-Based Medicine (EBM) in providing optimum clinical care. EBM involves systematic review and utilization of clinical research for informed decision-making, benefiting patients in disease management and treatment
7 views • 20 slides
Clinical Research Tools and Processes in EPIC for Healthcare Professionals
Clinical research professionals can utilize EPIC tools and processes to streamline study management, patient association, and research data tracking. The system enables the creation of study-specific research records, patient associations, and detailed study descriptions. From building research stud
1 views • 16 slides
General Clinical Research Unit (GCRU) Services Overview
The General Clinical Research Unit (GCRU) under Ridiane Denis offers support for a wide range of active protocols in various research areas like endocrinology, Alzheimer's, oncology, and more. GCRU provides clinical and laboratory services including sample processing, infusion, biopsy, urine analysi
0 views • 5 slides
Essential Aspects of the Clinical Interview in Psychology
Clinical interviews play a crucial role in the assessment conducted by clinical psychologists, showcasing essential qualities like validity, reliability, and clinical utility. Understanding the importance of feedback and honing general and specific skills as an interviewer are key components in cond
1 views • 17 slides
Deanery of Clinical Sciences Funding Challenge 2018 Launch Event
The Deanery of Clinical Sciences is offering a small grant opportunity for early career researchers within the clinical sciences field. The fund aims to support researchers' current studies and research projects with a maximum grant of £2,500. Applications are open to postgraduates and postdocs, an
3 views • 7 slides
Enhancing Human Subjects Research Through NIH Policy Changes
The National Institutes of Health (NIH) is implementing reforms and initiatives to improve the stewardship of research involving human subjects, particularly in the context of clinical trials. These changes include new forms for data collection, training in Good Clinical Practice, use of a single In
0 views • 13 slides
Clinical Research Guidelines and Regulations Overview
Clinical research encompasses various guidelines and regulations to ensure the protection of human subjects and the credibility of study results. Key aspects include Good Clinical Practice (GCP) standards, Title 45 of the Code of Federal Regulations (CFR) Part 46, and additional CFR sections for cli
0 views • 46 slides
Essential Elements of Clinical Trial Protocols
Understanding the key components of a clinical trial research protocol is essential for conducting successful studies. This includes identifying session objectives, discussing trial protocol contents, exploring observational study elements, and learning about reporting guidelines. Study objectives f
1 views • 25 slides
Clinical Judgement Test: Research Question Relevance and Study Design Assessment
This document presents a Clinical Judgement Test focusing on the relevance of a research question and the appropriateness of the study design. Candidates are evaluated on their critical review skills and ability to assess both strengths and weaknesses of the research methodology employed. The test a
0 views • 12 slides
Ohio Clinical Alliance: Transforming Clinical Experiences
The Ohio Clinical Alliance, through collaborative partnerships, aims to enhance clinical preparation for educators. The leadership team comprises various representatives and organizations committed to improving student learning. Their activities include retreats and meetings to ensure effective comm
0 views • 27 slides
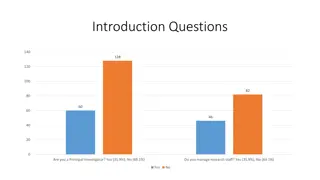
Survey Results on Research Training and Educational Support in Clinical Research Settings
This presentation showcases survey data on various aspects of research training and educational support in clinical research environments, including questions on being a Principal Investigator, managing research staff, training adequacy, study types, tenure at the University of Chicago, training pre
0 views • 35 slides
Safety Practices and Reporting in Clinical Research
Safety practices and reporting in clinical research are crucial for ensuring the rights, safety, and well-being of trial subjects. This includes monitoring safety, reporting adverse events promptly, and following regulatory requirements. Investigators play a vital role in assuring subject safety and
0 views • 33 slides
Enhancing Clinical Trials through ICH E6 Renovation
Explore the Clinical Trials Transformation Initiative's efforts in updating ICH E6 guidelines to improve global clinical trials. Learn about CTTI's findings, insights from stakeholders, and discussions on the necessary updates for ICH E6. Engage with industry experts and regulatory authorities to un
0 views • 65 slides
Pathways into Clinical Research: Linking Genes with Brain Development and Behavior
Explore the fascinating journey into clinical research as Dr. Emma Pagnamenta, a Research Manager at RCSLT, delves into linking genes with brain development and behavior. From special schools in Birmingham to mainstream and early years in Wandsworth and Oxfordshire, discover the importance of a clin
0 views • 16 slides
Perception and Awareness of Clinical Research in Trial Participants and the Public of Andhra Pradesh
This study focuses on understanding the perception and awareness of clinical research among trial participants and the general public in Andhra Pradesh. It highlights the importance of creating awareness about clinical research, previous study results, public attitudes towards clinical trials, and e
0 views • 24 slides
Understanding Clinical Psychology: Definition, Training, and Models
Clinical psychology, first introduced in 1907, encompasses a diverse field focusing on the study, assessment, and treatment of psychological issues. The APA Division 12 defines clinical psychology as integrating science, theory, and practice to understand and alleviate maladjustment. Training typica
0 views • 26 slides
Understanding Clinical Education Research: Insights and Methodologies
Clinical Education Research delves into various aspects such as teaching, learning strategies, learner wellbeing, professional regulation, and career paths within the clinical education domain. This research explores methods like surveys, interviews, realist reviews, and workplace interventions to e
0 views • 9 slides
Introduction to Clinical Trials and Important Terms
This content provides an overview of clinical trials, including the goals, phases, study funding, and important terms such as placebo. Clinical trials aim to study the impacts of health and disease, evaluate treatments, and ensure safety and effectiveness. The phases of clinical trials are explained
0 views • 27 slides
Comparison of Professional Behaviors in Clinical Education
Professional behavior characteristics play a crucial role in enhancing student learning during clinical education. This study examines the differences in reported importance and frequency of professional behaviors between credentialed and non-credentialed clinical instructors. The background outline
0 views • 28 slides
Enhancing Clinical Academic Collaboration Between Universities and NHS Trusts
Clinical academics play a crucial role in integrating clinical practice, research, and education within the NHS. Collaboration between universities and NHS trusts is key to ensure clinical academics address the right questions for patient care and societal benefit. Challenges include an aging clinic
0 views • 29 slides
NHMRC's New Grant Program: Advancing Clinical Trials and Research Funding
The NHMRC's latest grant program aims to enhance research in healthcare by focusing on clinical trials funding across four key streams: Investigator Grants, Synergy Grants, Ideas Grants, and Strategic and Leveraging Grants. The redistribution of funding will see a significant increase in support for
0 views • 12 slides
Understanding Clinical Trials: Phases, Types, and Definitions
Clinical trials play a crucial role in advancing medical research and treatment options. This comprehensive guide covers the basics of clinical trials, including their definition, phases, types, and key definitions like IND, IDE, NDA, and more. Discover how different phases of trials work, the vario
0 views • 16 slides
Ethics and Research Misconduct: A Course on Ethical Principles in Clinical Research
This course session covers essential topics related to ethics and research misconduct in clinical research, including definitions, ethical principles, key reports, and scenarios. The session emphasizes the importance of upholding ethical standards, such as respect, justice, beneficence, and accounta
0 views • 54 slides
Clinical Research Workflow Optimization Overview
Clinical research workflow optimization aims to streamline the process of subject enrollment, study approval, and Epic integration. It involves linking encounters to research studies, using automated tools for transactional data, and enhancing the research workqueue training. The process includes au
0 views • 21 slides