Understanding the Oxford-AstraZeneca Vaccine: Benefits, Risks, and Recommendations
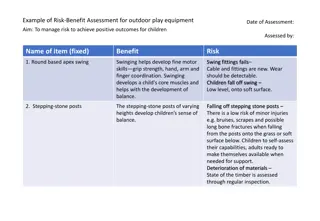
The Oxford-AstraZeneca vaccine has shown a likely but unproven link to cerebral venous sinus thrombus and other blood clotting issues. Despite this, the benefits of vaccination still outweigh the risks, with only approximately 4 cases per million resulting in death. Communication of the potential benefits and harms of the vaccine is crucial, especially in the context of low COVID-19 exposure risk. Primary care recommendations include offering the second dose of the vaccine to those who had no issues with the first dose, with clear guidelines on exceptional cases where the vaccine should be avoided.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
Summary of findings There is a likely but not yet proven link between AZ and cerebral venous sinus thrombus and other thrombus in major blood vessels In the UK there have been 79 cases reported after a first dose of AZ amongst 20,000,000 given, 19 resulted in death, approximately 4 per million As yet there is no proven link to age, gender of pre-existing medical conditions The conditions appear to be similar to heparin induced thrombocytopenia with low platelets, clots and presence of antibodies (so is an immune-mediated condition) Benefits of vaccination still outweighs risk (4 per million) To put in context with those who catch COVID 7.8% get pulmonary embolism 11.2% get a DVT 23% admitted to ITU get clots 1.6% will have a stroke 30% get low platelets In situations where there is low risk of exposure (with current circulating levels of COVID we are just below medium), the risk potentially outweigh benefits for under 30s, in terms of comparing possible ITU admission with COVID against significant side effects
Communicating the potential benefits and harms of the AZ COVID-19 vaccine (1)
Communicating the potential benefits and harms of the AZ COVID-19 vaccine (2)
Communicating the potential benefits and harms of the AZ COVID-19 vaccine (3)
What this means for primary care? Cerebral venous thrombosis rare syndrome 5-14 days post AZ vaccine, risk 4 per million Idiosyncratic event mediated by platelet factor 4 - immune mediated effect endothelial response in venous sinus, do not need to measure platelets before vaccination Second Doses Anyone who has had first dose AZ without any problems can be offered second dose as planned, JCVI states all those who have received a first dose of the AstraZeneca COVID-19 vaccine should continue to be offered a second dose of AstraZeneca COVID-19 vaccine, irrespective of age. MHRA are clear that the only individuals who should not have a second dose of AstraZeneca are those set out below: Administration of the COVID-19 Vaccine AstraZeneca in patients with a history of cerebral venous sinus thrombosis, acquired or hereditary thrombophilia, heparin-induced thrombocytopenia or antiphospholipid syndrome should only be considered when the potential benefit outweighs any potential risks. Patients who have experienced major venous and arterial thrombosis occurring with thrombocytopenia following vaccination with any COVID-19 vaccine should not receive a second dose of COVID-19 Vaccine AstraZeneca.
What this means for primary care? First doses for individuals 30 years of age and over and those who have underlying health conditions which put them at higher risk of severe COVID-19 disease JCVI has weighed the relative balance of benefits and risks and advise that the benefits of prompt vaccination with the AstraZeneca COVID-19 vaccine far outweigh the risk of adverse events for individuals 30 years of age and over and those who have underlying health conditions which put them at higher risk of severe COVID-19 disease. Therefore,for recipients in cohorts 1-9 aged 30 years and above who are scheduled to receive a first dose of AstraZeneca, vaccination should continue with consent obtained in line with the recommendations set out in the Green Book. Adults under 30 without underlying health conditions in Phase 1 JCVI guidance states There are some adults <30 without underlying health conditions who are in phase 1, who were prioritised due to an increased risk of exposure and/or to reduce the risk of passing the infection on to vulnerable individuals. This includes health and social care workers, unpaid carers and household contacts of immunosuppressed individuals. Acting on a precautionary basis, if these persons are still unvaccinated, it is preferable for them to be offered an alternative COVID-19 vaccine, if available. MHRA and JCVI are not saying that AZ vaccination should be stopped totally in this age group but preference that these younger age group offered an alternative, can still give AZ vaccination to under 30yrs with no other conditions just needs conversation with medical professional Expectation is that we would have been getting to the 18-30 cohort in July time
What this means for primary care? What is going to be happening in the next few days/week? First dose vaccinations at mass vaccination sites from Friday onwards for those aged 29yrs and younger will be cancelled and they will be contacted and asked to contact their GP to discuss the vaccination re risk benefit and if needed should be offered alternative vaccination (Pfizer or Moderna) when vaccine available GP practices can expect questions coming through on these issues as patients will be being advised by NHSE to contact GP surgeries for advice, please do share this information with your teams Helpful advice leaflet that can be shared with patients can be found here: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/976880/ PHE_COVID-19_AZ_vaccination_guide.pdf (publishing.service.gov.uk)