Wharton Printing Transition Overview
A detailed overview of the transition of Wharton Printing, including organizational assessment, transition issues, Campus Copy procedures, and the role of Maximum Graphics as the sole provider of stationery and business cards. Changes in printing expenses, paper ordering, and departmental processes are also highlighted.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
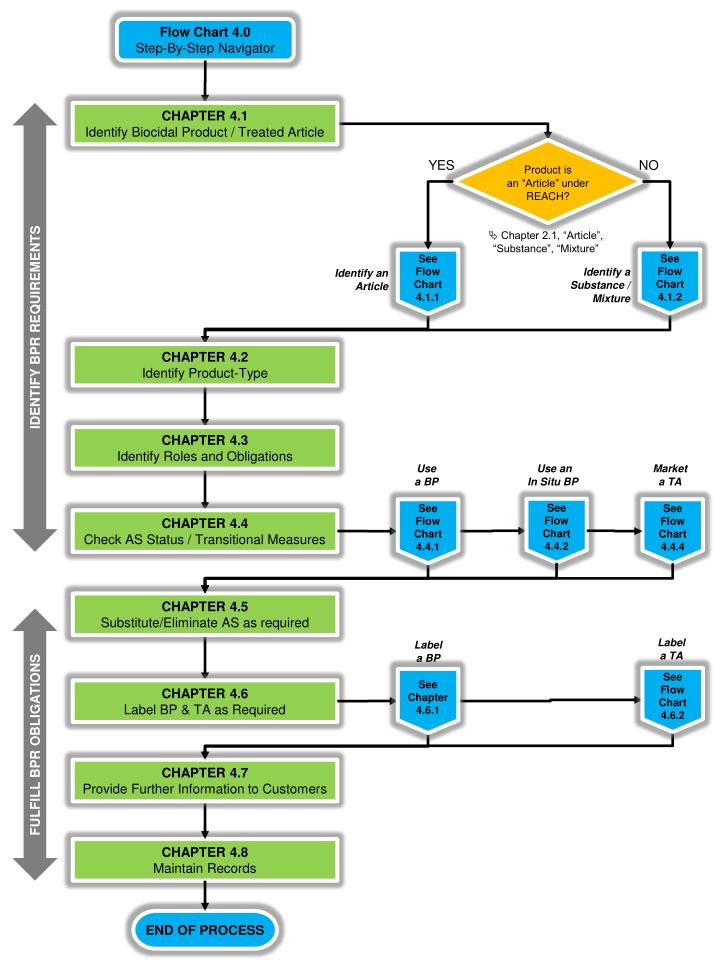
Flow Chart 4.0 Step-By-Step Navigator CHAPTER 4.1 Identify Biocidal Product / Treated Article YES NO Product is an Article under REACH? IDENTIFY BPR REQUIREMENTS Chapter 2.1, Article , Substance , Mixture See Flow Chart 4.1.2 See Flow Chart 4.1.1 Identify a Substance / Mixture Identify an Article CHAPTER 4.2 Identify Product-Type CHAPTER 4.3 Identify Roles and Obligations Use a BP Use an In Situ BP Market a TA See Flow Chart 4.4.2 See Flow Chart 4.4.1 See Flow Chart 4.4.4 CHAPTER 4.4 Check AS Status / Transitional Measures CHAPTER 4.5 Substitute/Eliminate AS as required Label a TA Label a BP FULFILL BPR OBLIGATIONS See Flow Chart 4.6.2 See Chapter 4.6.1 CHAPTER 4.6 Label BP & TA as Required CHAPTER 4.7 Provide Further Information to Customers CHAPTER 4.8 Maintain Records END OF PROCESS
Flow Chart 4.1.1 Identify BP / TA REACH Articles Is YES NO Product is Biocidal Product for generation of in situ active substance(s) finished product used to generate active substance(s)? Chapter 2.1, In situ generated active substances Is YES NO finished product treated short-term with biocidal product? Product is Treated Article without biocidal function Chapter 2.1, Treated with Does YES Product is neither BP nor TA (Out of scope of BPR) NO finished product intentionally incorporate biocidal product? Chapter 2.1, Intentionally Incorporating END OF PROCESS Does YES product have a biocidal property or function relevant to finished product? NO Chapter 2.1, Biocidal Property , Biocidal Function YES NO Product is Treated Article with biocidal property or biocidal function Does Product is Biocidal Product product have a primary biocidal function? Chapter 2.1, Primary biocidal function Go to Chapter 4.2
Flow Chart 4.1.2 BP / TA Identification: Substances & Mixtures Is Active YES NO Substance generated in situ from precursors? Precursor combination is Biocidal Product Chapter 2.1, In situ generated active substances Does NO YES product contain AS and have biocidal function rel- evant to finished product? Product is Biocidal Product Chapter 2.1, Biocidal function Is product YES NO Product is neither BP nor TA (Out of scope of BPR) treated with, or does it intentionally incorporate, biocidal product? Product is Treated Article Chapter 2.1, Intentionally Incorporating , Treated with END OF PROCESS Go to Chapter 4.2
Flow Chart 4.4.1 Use a Biocidal Product Is AS NO YES on the Annex I list under Categories 1 to 5 or 7? AS may be used in BPs for all PTs Chapter 4.4.4 Is AS NO YES on the Annex I list under Category 6? Chapter 4.4.4 Is AS/PT approved on the Union List? NO YES Chapter 4.4.5 YES NO Was AS/PT in the Review Programme before 1 Sep 2016? Chapter 4.4.6 Is supplier listed for the AS/PT combination on the Article 95 List? AS may be used for the specified PT (pending approval decision if AS/PT is in the Review Programme) YES NO Chapter 4.4.10 AS may not be used in a BP since1 Sept 2015, unless a new approval application is approved Go to Chapter 4.5
Flow Chart 4.4.3 Market a Treated Article NO YES Is AS listed in BPR Annex I? Chapter 4.4.4 TA may be marketed without time limit Is AS NO YES approved on the Union List for the relevant PT? Chapter 4.4.5 Is AS/PT in YES NO the Review Programme before 1 Sep 2016? Chapter 4.4.6 TA may not be marketed after 1 Mar 2017, unless a new application is approved NO YES Is a non-approval decision on the AS/PT made before 1 Sep 2016? Chapter 4.4.8 YES NO Is non-approval decision on the AS/PT made after 1 Sep 2016? TA may not be marketed after 180 days after non-approval decision, unless a new application is approved Chapter 4.4.8 TA may be marketed, pending approval decision Go to Chapter 4.5
Flow Chart 4.6.2 Treated Article: Labelling Obligations NO Does the TA YES intentionally incorporate a Biocidal Product? Chapter 4.6.3 NO Is a claim YES made regarding the biocidal properties of the TA? Provide labelling that includes : Statement that the TA incorporates biocidal products Names of all active substances contained in the biocidal products Names of all nanomaterials contained in the biocidal products; Relevant instructions and any precautions for use. Chapter 2.1, Claim Does an AS used require labelling as a condition of approval? YES NO Chapter 4.6.4 NO YES Has a biocidal property of the TA been substantiated? Include on labelling: Biocidal property attributed to the treated article Chapter 4.6.5 TA may be placed on the market with labelling TA may be placed on the market without labelling Go to Chapter 4.7