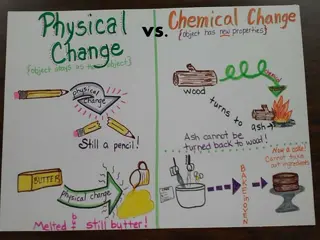Understanding Matter: Physical and Chemical Changes Explained
Explore the nature of matter through the concepts of physical and chemical changes. Learn to distinguish between physical changes where the form or shape of a material alters without new substances forming, and chemical changes which result in the creation of new compounds. Discover reactivity in chemical reactions and the concept of sublimation as a solid transitions directly into a gas. Watch educational videos and examples to deepen your understanding of these fundamental scientific principles.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
VOCABULARY ADVANCED CLASS Matter Unit 1.3 Week of August 22, 2016 You will need 4 index cards
S8P1. Students will examine the scientific view of the nature of matter. e. Distinguish between changes in matter as physical (i.e., physical change) or chemical (development of a gas, formation of precipitate, and change in color).
eactivity, combustability, precipitate, oxidation) chemical change, physical change, sublimation,
PHYSICAL CHANGES Examples: crumpling a sheet of aluminum foil melting an ice cube casting silver in a mold breaking a bottle A material may change shapes or forms while undergoing a physical change, but no chemical reactions occur and no new compounds are produced. the identity of the matter does not change. Easier or possible to change back.
Examples: burning wood souring milk mixing acid and base digesting food cooking an egg heating sugar to form caramel baking a cake rusting of iron CHEMICAL CHANGE A Material changes and produces a new substance. A new compound (product) results from a chemical change as the atoms rearrange themselves to form new chemical bonds. Difficult or impossible to reverse.
CRASH COURSE CHEMICAL CHANGES HTTPS://WWW.YOUTUBE.COM/WATCH?V=37PIR0EJ_SE
REACTIVITY Reactivity in science refers to how various chemicals participate in chemical reactions when they are exposed to other substances. A chemical that reacts easily with other substances is considered highly reactive. Examples: One example of a reactive material is magnesium, which burns brightly when heated. In contrast, platinum does not burn when heated and it is considered non-reactive. Some chemicals react together so strongly that they create a new substance called a compound. A common example of a compound formed through reactivity is water, formed when hydrogen and oxygen react.
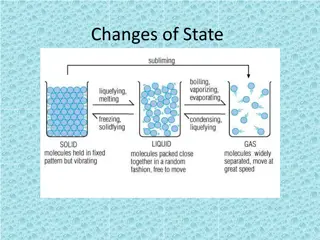
SUBLIMATION When a solid changes directly to a gas, skipping the liquid phase. https://www.youtube.com/watch?v=PaOFohqjZ1s