Understanding Changes in Matter: Physical vs. Chemical
Explore the concepts of physical and chemical changes in matter through engaging activities, discussions, and demonstrations. Learn to differentiate between the two types of changes, classify chemical equations, and understand the nuances between physical and chemical transformations. Delve into how substances alter forms or create new properties, enhancing your grasp of stoichiometry, solution chemistry, and acids and bases.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
Living By Chemistry SECOND EDITION Unit 4: TOXINS Stoichiometry, Solution Chemistry, and Acids and Bases
Lesson 70: Spare Change Physical Versus Chemical Change
ChemCatalyst A chunk of blue, solid cobalt (II) chloride, CoCl2, is placed into some water. The water turns from colorless to pink in color. 1. What type of change has taken place? How do you know? 2. Was something new made? Explain.
Key Question How are changes in matter classified?
You will be able to: define physical and chemical change and explain the gray areas between them classify chemical equations as representing physical changes or chemical changes
Prepare for the Demonstration Work as a class.
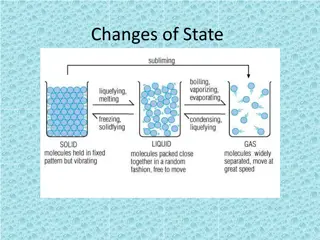
Discussion Notes Physical changes are changes in the appearance or form of a substance. Chemical changes produce new substances with new properties. Physical change: A change in matter in which a substance changes form but not identity. Chemical change: A change in matter that results in the formation of a new substance or substances with new properties.
Discussion Notes (cont.) CoCl2(s) CoCl2(aq) CoCl2(aq) + Ca(OH)2(aq) Co(OH)2(s) + CaCl2(aq) It is not always possible to distinguish between physical and chemical change based on observations alone. It is possible to argue that dissolving a substance in water changes the properties of that substance.
Discussion Notes (cont.) Ionic compounds do not dissolve in the same way as molecular solids. The dissolving of ionic solids can be shown with a type of equation that stresses the formation of ions in solution. CaCl2(s) CaCl2(aq) CaCl2(s) Ca2(aq) + 2Cl (aq)
Wrap Up How are changes in matter classified? Chemical changes involve the formation of new substances. Physical changes, such as phase changes, involve a change in form. Dissolving generally is considered a physical change, but it has something in common with chemical change as well. Chemical equations often provide more straightforward information about the type of change than do mere observations.
Check-In Does this chemical equation describe a physical change or a chemical change? Explain how you can tell. C17H17O3N(s) + 2C4H6O3(l) C21H21O5N(s) + 2C2H4O2(l)