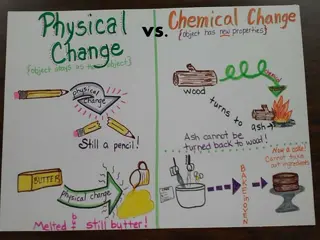Understanding Physical and Chemical Properties of Matter
Explore the distinction between physical and chemical properties of matter in Chapter 2, Section 2. Physical properties can be observed without changing the substance's identity, such as color and density, while chemical properties require altering the substance to observe characteristics like reactivity. Examples and explanations of physical and chemical properties are provided, aiding in a deeper comprehension of the nature of matter.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
Physical versus Chemical Properties Chapter 2 Section 2 Describing matter
Reviewing MATTER Matter: anything that has mass and takes up space Mass the amount of matter in something Volume the amount of space something occupies Which of the following is matter? A car? A box? You?
What is a property? Property: a characteristic of a substance that can be observed
Physical Property Physical property: a property that can be observed without changing the identity of the substance. Examples: luster malleability: the ability to be hammered into a thin sheet ductility: the ability to be stretched into a wire melting point boiling point density solubility specific heat
Physical Properties Color Shape Size Density Melting Point Boiling Point
Example of Physical Property The physical properties of sodium metal can be observed or measured. It is a soft, lustrous, silver-colored metal with a relatively low melting point and low density. Hardness, color, melting point and density are all physical properties.
Special Physical Properties Melting point: the temperature at which a substance changes from a solid to a liquid at a given pressure water = 0oC Boiling point: the temperature at which a substance changes from a liquid to a gas at a given pressure water = 100oC
Thermal State Density Ductility Malleability Solubility
Chemical Properties Chemical property: a property that can only be observed by changing the identity of the substance
Chemical Properties Examples of Chemical Properties Reactivity with oxygen Nonreactivity with oxygen Flammability Nonflammability
Chemical Properties Cars, Automobiles; Old Rusty Car - Pictures, Photos, Photographs
Comparison of Physical and Chemical Properties
Density Density is the amount of mass per unit of volume. Density can be used to identify a substance. The density of water is 1.0g/mL
Density Calculations Calculations: D = m/V Ex: A cube has a mass of 2.8 g and occupies a volume of 3.67 ml. Would this object float or sink in water? Mass = 2.8 g D = 2.8g/3.67 mL= 0.76 g/mL This object would float in water because its density is less than water (1.0 g/mL). Volume = 3.67 mL
More Density Calculations Ex: A liquid has a mass of 25.6 g and a volume of 31.6 mL. Use the table below to identify the substance. Substance Density (g/mL) M=25.6 g D = 25.6 g/31.6 mL D= 0.81 g/mL The substance is ethanol. V=31.6 mL Mercury 13.6 Water 1.00 Ethanol 0.81
Physical Change Physical change is the change that affects one or more physical properties of a substance. Imagine breaking a piece of chalk into two pieces. What are you changing? What is not being changed? Physical changes do not change the identity of the matter involved
Physical Change Freezing water for ice cubes Sanding a piece of wood Cutting your hair Crushing an aluminum can Bending a paper clip Mixing oil and vinegar
Chemical Change Chemical change happens when two or more substance are changed into one or more new substances with different properties. Properties of a substance describe which chemical changes will or will not happen Chemical change and properties are not the same, a change is the process in which it changes
Chemical Change Examples of Chemical Changes Soured milk Effervescent tablets Statue of Liberty Baking a cake
Chemical Change Clues that chemical change has occurred Changes in color Heat Fizzing and foaming Production of sound or light