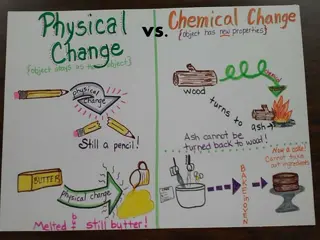Exploring Matter and Physical/Chemical Changes in Science Class
Delve into the world of matter and its transformations through engaging activities such as classifying elements, compounds, and mixtures using nuts, bolts, and washers. Understand the concepts of mass, space, and the different types of matter through hands-on learning experiences in this interactive session. Sign your safety contract, review the syllabus, and get ready to discover the fascinating properties of matter in your next lab session!
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
INTRO TO MATTER AND P/C CHANGES
DRILL Take out your signed safety contract and signed syllabus sheet and place them in the correctly labelled bin **You keep the rules paper, I only need the signed copy for the safety contract
SAFETY CONTRACTS I NEED THIS BEFORE YOU ARE ABLE TO DO ANY LAB!!!!!!! Lab = Next Class
AGENDA Drill Syllabus Review Intro to Matter Nuts and Bolts Activity Intro to Matter Graphic Organizer/Practice Sorting Activity Assign Homework
SYLLABUS REVIEW Take Out Your Phones!!!!
MATTER Anything that has mass and takes up space
INTRODUCTION TO MATTER There are 9 petri dishes located around the room with different nuts, bolts, and washers Your task is to classify each of them by representing an element, a compound, a mixture of elements, a mixture of compounds, or a mixture of elements and compounds. You will then define what you believe an element, a compound, and a mixture is
LOGISTICS Please wait until I say to move to go to the next Petri dish You will have 1-2 minutes at each station At the end you will have time to go back to any Petri dish you would like a second look at
AT THE END Go back to any petri dish you want to look at again Complete the bottom portion where you are to define: Element Compound Mixture
SUGAR (C12H22O11) E Element C Compound H Homogeneous Mixture M Heterogeneous Mixture
PEPPERONI PIZZA E Element C Compound H Homogeneous Mixture M Heterogeneous Mixture
SUGAR WATER E Element C Compound H Homogeneous Mixture M Heterogeneous Mixture
SILVER E Element C Compound H Homogeneous Mixture M Heterogeneous Mixture
A DILUTE SOLUTION OF HYDROCHLORIC ACID E Element C Compound H Homogeneous Mixture M Heterogeneous Mixture
GLASS OF ORANGE JUICE E Element C Compound H Homogeneous Mixture M Heterogeneous Mixture
SALT E Element C Compound H Homogeneous Mixture M Heterogeneous Mixture
QUESTION Is it easier to prove that an unknown substance is an element or a compound?
GROUPING ACTIVITY Grab a giant whiteboard and your marker bin With your group, read the statement on the sheet of paper Categorize them into multiple groups (you decide how many) and label them
GROUPING MORE DETAIL Walk around the room for 1 minute and see how other groups categorized their statements Ask a group member, if you are unsure If needed, take one minute to rearrange yours if you want to make any changes
LETS TALK Boiling water Dissolving sugar into water Leaves changing color in the fall
PHYSICAL CHANGES Crushing a can Melting an ice cube Boiling water Breaking glass Mixing sand and water Dissolving sugar into water Shredding paper Chopping wood
CHEMICAL CHANGES Rusting of iron Combustion (burning) of wood Cooking an egg Explosion of fireworks Milk going sour Baking a cake Leaves changing in the fall Metabolism of food in the body
LAWS OF MATTER Law of Conservation of Matter: Matter cannot be created or destroyed Law of Definite Proportions: A compound contains the same proportion of elements by mass
PHYSICAL CHANGES Definition Changes in size, shape, state, or appearance Matter stays the same Examples Breaking Bending Cutting Melting Boiling
CHEMICAL CHANGES Definition Matter changes to a different kind of matter Make something new Examples Rusting Cooking Burning Reacting
DEMO Lighting Magnesium Ribbon 2Mg + O2 2MgO
When magnesium burns, it is actually reacting with oxygen in the air and not with fire. Fire is what we call the heat and light produced when things burn. Magnesium reacts with oxygen to make a compound called magnesium oxide. 2Mg + O2---> 2MgO The bright light results because this reaction produces a lot of heat. When the magnesium gets really hot, it emits energy in the form of light (heat is also how the sun produces light). Since it emits all visible wavelengths at a relatively equal rate, the light looks white-ish. Be careful when burning magnesium though, because it also produces ultra-violet (UV) light which can damage your eyes if you stare at it for too long. The process of hot objects emitting light is known as incandescence (like an incandescent light bulb). The spectrum of light emitted by hot objects is known as black body radiation.
HOMEWORK Signed syllabus and safety contract (if you didn t turn it in) REMEMBER: No Contract NO LAB!!! Wear closed-toe shoes Write procedure and draw data table for lab