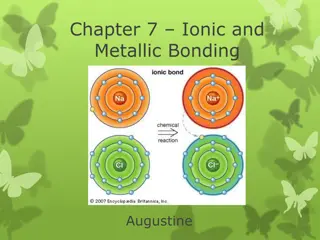
Understanding Ionic and Metallic Bonding: Valence Electrons, Octet Rule, and Ion Formation
Explore the essential concepts of ionic and metallic bonding, focusing on valence electrons, electron dot structures, the octet rule, cations, anions, and ion formation. Discover how atoms achieve stability through electron transfer, and learn to write electron configurations for various ions.
9 views • 52 slides
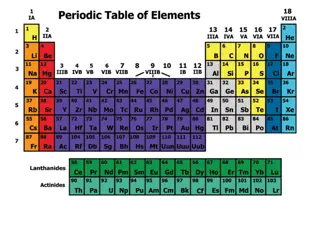
Understanding the Periodic Table and Chemical Bonds in Physical Science
The periodic table organizes elements based on their properties, with rows representing periods and columns representing groups. Mendeleev's early table laid the foundation for predicting undiscovered elements. Today's periodic table orders elements by atomic number, showcasing the periodic law and
3 views • 15 slides
Evolution of Mathematical Theories and Proof Systems
Development of mathematical theories such as model theory, proof theory, set theory, recursion theory, and computational complexity is discussed, starting from historical perspectives with Dedekind and Peano to Godel's theorems, recursion theory's golden age in the 1930s, and advancements in proof t
1 views • 29 slides
Maximum Price Calculation for Callable Bond with Annual Yield Requirement
A 20-year callable bond example is provided with a $1000 face value and 3% annual coupons, callable at different redemption values over specific years. The task is to determine the maximum price a buyer should pay to achieve a minimum annual yield of 5%. The calculation involves identifying the time
0 views • 33 slides
Understanding Callable Bonds in Bond Investments
Callable bonds are a type of bond where the issuer reserves the right to redeem the bond at different times, potentially at different values. Investors face uncertainty about when the bond will be redeemed, but it can only be called at predetermined times. Common questions revolve around determining
0 views • 13 slides
Understanding Hybridization in Organic Chemistry
Delve into the complexities of the Lewis octet model and the insights provided by Linus Pauling's localized valence bond hybridization model to explain bond shapes in molecules, reactivity trends, and electron distribution in double and triple bonds. Discover how hybridization transforms atomic orbi
0 views • 22 slides
Granny's Tree Climbing - A Poetic Adventure by Ruskin Bond
In this whimsical poem by Ruskin Bond, we are introduced to Granny, a sprightly sixty-two-year-old who defies convention by indulging in her lifelong passion for tree climbing. Despite admonishments from her family, Granny remains steadfast in her joy as she embarks on a delightful tree-climbing esc
0 views • 19 slides
Bringing Up Kari: A Tale of Compassion and Bond Between a Boy and an Elephant
Revolving around a young elephant named Kari and a nine-year-old boy, this heartwarming story explores the strong bond they share. Through adventures and challenges, the narrator teaches Kari valuable skills, nurturing a deep connection built on compassion and understanding. As Kari grows, so does t
0 views • 7 slides
Chemistry Concepts: Valence Electrons, Ion Charges, and Ionic Compounds
Explore various key concepts in chemistry such as valence electrons in magnesium, Lewis Dot structure for silicon, charges on ions like strontium, formation of ions to achieve noble-gas electron configuration, elements forming ions with specific charges, and the octet rule. Learn about the character
1 views • 48 slides
Understanding the Theory of Firms: Neoclassical vs. Modern Approaches
The theory of firms is explored through the Neoclassical and Modern perspectives. Neoclassical theory focuses on profit maximization, while Modern theory delves into managerial, principal-agent, and transaction cost theories. The discussion covers criticisms of Neoclassical theory and the essential
1 views • 79 slides
Understanding Valence Bond Theory in Chemistry
Valence Bond Theory (VBT) explains the formation of covalent bonds through overlapping of valence orbitals, introducing Sigma and Pi bonds. This theory is essential to understand the geometry and stability of complex molecules.
1 views • 19 slides
Understanding the Impact of Temperature on Fermi Level in Semiconductors
The Fermi level plays a crucial role in determining the behavior of electrons in semiconductors at different temperatures. As temperature increases, the Fermi level shifts, affecting the generation of free electrons and holes in the valence and conduction bands. In intrinsic semiconductors, electron
2 views • 53 slides
Understanding Valence Electrons in Chemistry
Explore the concept of valence electrons with definitions, diagrams, and explanations of how electrons are located in the electron cloud. Learn about the importance of valence electrons in determining an element's reactivity and stability. Discover how atoms gain or lose electrons to achieve a full
0 views • 13 slides
Understanding Valence Electrons and Ionic Charges in Elemental Bonding
Valence electrons play a crucial role in the formation of ions as elements combine. Nonmetals gain electrons to become negatively charged ions, while metals lose electrons to become positively charged ions. This process leads to the creation of electrically attractive elements open for bonding. The
0 views • 17 slides
Understanding Valence Electrons in Atoms and the Periodic Table
Explore the concept of valence electrons in atoms, crucial for chemical bonding. Learn to define valence electrons, understand their role, and draw electron-dot structures for atoms. Practice identifying valence electrons in various elements and creating electron-dot structures in this educational c
1 views • 12 slides
Understand Molecular Structures with Lewis Dot Symbols
Explore the world of molecular structures with Lewis dot symbols in this chemistry unit. Learn about valence electrons, covalent bonding, and the HONC 1234 rule through engaging activities and discussions. Create accurate structural formulas and describe bonding in molecular substances. Get ready to
0 views • 13 slides
Evolution of Light Theory: From Wave Theory to Quantum Theory
At the turn of the century, the discovery of the photoelectric effect challenged the wave theory of light, leading to the development of the quantum theory by Max Planck and Albert Einstein. This new theory introduced the concept of discrete energy units known as quanta, bridging the gap between wav
1 views • 62 slides
Exploring the Human-Animal Bond in Veterinary Medicine
Delve into the significance of the human-animal bond in veterinary medicine, highlighting the mutually beneficial relationship between animals and humans over time. Understand how this bond has evolved, the role of veterinary assistants in fostering this connection, and the changing dynamics of huma
0 views • 26 slides
Understanding Alkynes in Organic Chemistry
Alkynes are unsaturated hydrocarbons with at least one triple bond, following a molecular formula of CnH2n-2. This group of compounds is discussed in Chapter three, covering topics like structure, hybridization, common naming, physical properties, preparation, and reactions. The sp hybridization of
1 views • 20 slides
Understanding the Periodic Table and Valence Electrons
Dmitri Mendeleev's organization of elements into the periodic table, the layout of periods and groups, and the significance of valence electrons in determining chemical reactivity are explored in this informative content. Discover how valence electrons relate to group numbers and learn about excepti
0 views • 31 slides
Understanding the Periodic Table: Mendeleev's Discovery and the Role of Valence Electrons
Russian chemist Dmitri Mendeleev's groundbreaking work in 1869 led to the creation of the periodic table, showcasing a pattern in the arrangement of elements by increasing atomic mass. Discover how Mendeleev's table evolved, the significance of Henry Moseley's contribution in 1914, and the importanc
0 views • 61 slides
Exploring Growth and Opportunities in the Green Bond Markets
Delve into the burgeoning Chinese green bond market with insights on green bonds growth, sector allocations, Latin American issuance trends, sustainable investment needs in Latin America, China's green bond growth, and global green bond market size comparisons. Discover the significance of Green Pan
0 views • 14 slides
Understanding Extrinsic Semiconductors: Fermi Level and Doping Effects
Extrinsic semiconductors play a crucial role in modern electronics by allowing controlled addition of impurities to tailor conductivity. The Fermi level in extrinsic semiconductors shifts based on the number of electrons and holes in the conduction and valence bands, influencing conductivity. Doping
0 views • 14 slides
Arlington ISD Citizens Bond Oversight Committee Report August 2016
The Arlington ISD Citizens Bond Oversight Committee (CBOC) was established to provide transparency, enhance public confidence, provide findings and recommendations to the Board of Trustees, monitor progress of the 2014 Bond program, and find ways to maximize the bond's potential. The committee compr
0 views • 27 slides
Understanding Valence Electrons and Lewis Dot Diagrams
Explore the concept of valence electrons and Lewis dot diagrams in chemistry. Learn how to identify the number of protons, neutrons, and electrons in an element using Bohr model drawings. Discover the significance of valence electrons in bonding and how to determine the number of valence electrons f
0 views • 37 slides
Bonded Warehouse Facility and New Bond Process
Bonded warehouse facilities offer a convenient way for traders and industrialists to store goods without paying duties. The process involves applying for a new bond with specific requirements and inspections by officers. Necessary documents and approvals are essential to secure the bond premises, en
0 views • 31 slides
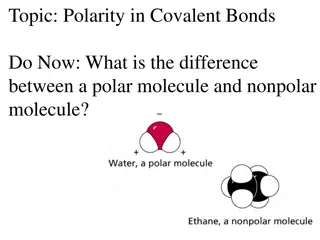
Understanding Polarity in Covalent Bonds
The difference between a polar molecule and a nonpolar molecule lies in the distribution of electrons. A polar molecule has an asymmetric electron distribution due to a significant difference in electronegativity, while a nonpolar molecule has a symmetric electron distribution. You can predict polar
0 views • 15 slides
Understanding the Bond Market: Maturity, Yield, and Pricing
Financial markets facilitate borrowing and lending, influencing interest rates, stock prices, and bond prices. Bonds promise future payments in exchange for current prices, while stocks offer ownership rights and dividends. The bond market involves maturity dates, coupon rates, and yield to maturity
0 views • 17 slides
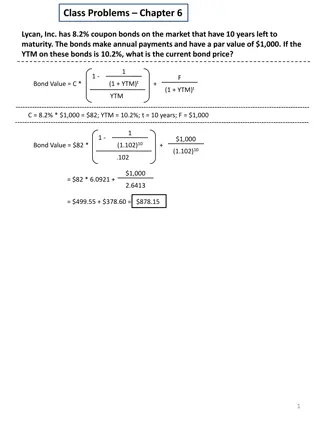
Understanding Callable Bonds and Bond Amortization
Callable bonds provide issuers with the right to redeem the bond before maturity under certain conditions. This article discusses the concept of callable bonds, bond amortization, premium bonds, discount bonds, and provides examples of calculating bond values based on specific scenarios.
0 views • 12 slides
Understanding Bond Premiums and Their Impact on Financial Decision-Making
Bond premiums occur when bond prices increase in the secondary market due to a drop in market interest rates. They can be used for approved project costs, debt service, or reducing bond principal. Utilizing bond premiums wisely can lead to cost savings for taxpayers. Learn how bond premiums can affe
0 views • 5 slides
Understanding Bond Valuation Models and Yield Relationship
Explore the fundamentals of bond valuation, including the present value model and the yield model, to understand how bond prices are determined based on factors like market price, coupon payments, and yield to maturity. Learn about the price-yield curve, convexity, and how to calculate expected yiel
0 views • 25 slides
Theories of Interest in Microeconomics II
Explore various theories of interest in economics, including the Classical Theory, Liquidity Preference Theory by Keynes, Productivity Theory, Abstinence Theory, Time-Preference Theory, Fisher's Time Preference Theory, and the Loanable Fund Theory. These theories offer different perspectives on the
0 views • 6 slides
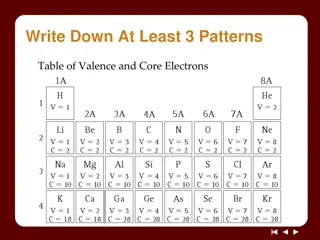
Understanding Electron Arrangements in Atoms
Explore the concepts of electron arrangements in atoms through activities and discussions on valence and core electrons, shell models, and patterns in the periodic table. Discover how the number of electrons in shells relates to element properties and learn to create shell model diagrams. Dive into
0 views • 14 slides
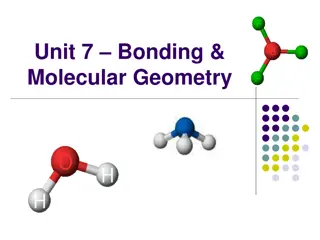
Understanding Chemical Bonds and Molecular Geometry
Chemical bonds are the forces that hold atoms together, with valence electrons playing a crucial role. Ionic bonds involve complete electron transfer between metals and nonmetals, while covalent bonds see electrons being shared. Lewis dot diagrams help in visualizing the valence electrons of atoms,
0 views • 68 slides
Bond Analysis and Valuation Techniques by Binam Ghimire
Explore the analysis and valuation of bonds in-depth with a focus on present value techniques, bond yields, and calculating future bond prices. Understand the process of pricing a bond by determining cash flows, coupon payments, and par value. Dive into the calculations involved in determining the p
0 views • 40 slides
Bond Pricing and Yield Calculation Examples
The provided content illustrates calculations for bond pricing and yield in various scenarios. It covers topics such as determining current bond prices based on coupon rates, yield to maturity, and time to maturity. Additionally, it explores scenarios with different bond characteristics like annual
0 views • 5 slides
Understanding Bond Valuation and Types
Explore the world of bond valuation, from the definition of bonds to the different types such as zero-coupon, coupon, self-amortizing, and perpetual bonds. Learn about bond issuers, including the US government and agencies, and delve into the specifics of US government bonds like Treasury Bills, Not
0 views • 39 slides
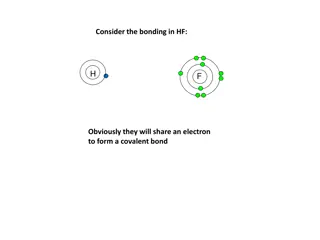
Understanding Bonding in HF Molecule
In HF bonding, hydrogen and fluorine share an electron to form a covalent bond. Fluorine, being more electronegative, attracts the bonding electrons more, resulting in a polar covalent bond. If hydrogen was less electronegative, the bonding electrons would shift further towards fluorine until an ion
0 views • 11 slides
Understanding Bond Lengths and Strengths in Chemistry
Bond lengths represent the critical distance between bonded atoms for maximum stability, while bond strengths are measured through dissociation energy and average bond energy. Methods for measuring bond lengths include X-ray diffraction and spectroscopic methods, with bond energies reflecting the st
0 views • 38 slides
Understanding Ionic Bonds: Valence Electrons and Ion Formation
Exploring the concepts of valence and core electrons, Noble Gas Envy Ions, and the formation of ions through electron transfer between metal and nonmetal atoms. Learn about cations and anions, ion charges based on the periodic table, and the relationship between ion charge and valence electrons.
0 views • 13 slides