Canizzaro Reaction in Organic Chemistry: Experiment and Applications
The Canizzaro reaction involves the disproportionation of aldehydes in the presence of a strong base to produce an alcohol and a carboxylic acid. This experiment, supervised by Lecturer Israa Radhi, explores the mechanism and practical application of the reaction. Benzyl alcohol and benzoic acid, pr
1 views • 7 slides
Aldol Condensation Reaction: Preparation of Chalcones
Chalcones are important unsaturated aromatic ketones that serve as biogenetic precursors of flavonoids and isoflavonoids. They have various medicinal and pharmaceutical applications due to their biological activities. Chalcones are easily synthesized compounds with potential therapeutic uses, making
2 views • 13 slides
Understanding the Seliwanoff Color Reaction and its Significance
The Seliwanoff color reaction, discovered by Russian chemist Feodor Feodorovich Selivanov, is used to differentiate between aldoses and ketohexoses based on their dehydration and reaction with resorcinol in acidic conditions. Ketoses like fructose react faster than aldoses like glucose, leading to a
3 views • 20 slides
Aspen Simulation of Steam Reforming and Haber-Bosch Processes in Kinetics Reactors
Aspen simulation showcases the kinetics reactors for steam reforming of natural gas and the Haber-Bosch process for ammonia production. Steam reforming is highly endothermic, producing hydrogen and CO, while the Haber-Bosch process is exothermic, crucial for ammonia synthesis. The RPlug reactor and
3 views • 26 slides
Understanding the Diels-Alder Reaction in Practical Organic Chemistry
The Diels-Alder reaction is a fundamental method in organic chemistry for producing cyclic organic compounds by combining a conjugated diene with an alkene. This reaction, named after Otto Diels and Kurt Alder, involves the formation of a six-membered ring with specific bond rearrangements. Conjugat
4 views • 15 slides
Chemical Kinetics: Understanding Reaction Rates and Factors
Chemical kinetics is a branch of physical chemistry that explores the velocity and factors influencing chemical reactions. It studies how reactants transform into products, considering conditions like temperature, pressure, and reactant concentrations. Factors affecting reaction rates include the na
7 views • 24 slides
Cannizzaro Reaction
The Cannizzaro reaction is a chemical reaction involving the base-induced disproportionation of non-enolizable aldehydes to form a primary alcohol and a carboxylic acid. Discover more about this reaction, its history, mechanism, and variants like the Cross Cannizzaro reaction and Intramolecular Cann
1 views • 20 slides
Benzoin Condensation: A Name Reaction Explained by Dr. Atul Kumar Singh
Benzoin condensation is a classic organic reaction where aromatic aldehydes self-condense to form α-hydroxy ketones. Dr. Atul Kumar Singh, an Assistant Professor of Chemistry, details the mechanism and the specific catalytic properties of cyanide in this reaction. The reaction involves refluxing th
0 views • 6 slides
Investigating Impact of Practice on Human Reaction Time Through Ruler Drop Test
This practical investigation focuses on determining if practice can reduce human reaction times by conducting a ruler drop test. Participants use their weaker hand to catch a ruler dropped by their partner, aiming to improve their reaction time with practice. The experiment explores how athletes can
0 views • 7 slides
Understanding Chemical Kinetics: Rates of Reactions and Factors Influencing Them
Chemical kinetics delves into the speed of chemical reactions and the factors that influence reaction rates. This field explores how collisions between atoms, ions, or molecules drive chemical reactions, as well as the role of catalysts, reactant concentration, temperature, and surface area. By unde
0 views • 32 slides
Understanding Antigen-Antibody Precipitation Reaction in Microbiology
Antigen-antibody precipitation reaction involves the formation of insoluble products when a soluble bivalent antibody interacts with a soluble antigen. This reaction leads to the formation of a visible precipitate known as a lattice. The mechanism of precipitation, including the prozone phenomenon,
0 views • 20 slides
Kinetic Reaction of Sulphite and Iodate - Landolt Reaction Overview
The kinetic reaction of sulphite ions and iodate in the Landolt reaction is a fascinating chemical process where slow and fast reactions occur sequentially, resulting in a visually striking color change. By monitoring the induction period between the two reactions, one can observe the formation of h
0 views • 9 slides
Exploring Enzyme Kinetics for Understanding Chemical Reactions
Enzyme kinetics is a vital discipline focusing on the rate of enzyme-catalyzed reactions and how they respond to varying conditions. Reactions are classified based on reactant concentration influences. Zero, first, second, and third order reactions are distinguished, with examples like first-order r
0 views • 31 slides
Understanding Chemical Kinetics: Rates, Reactions, and Mechanisms
Chemical kinetics involves studying reaction rates, rate laws, stoichiometry, and factors affecting reaction speed. This branch of chemistry delves into determining reaction orders, rate constants, and activation energies using various methods. Different types of rates, such as initial, instantaneou
2 views • 68 slides
Understanding the Kinetics of Fast Reactions in Chemistry
Kinetic methods involve measuring analytical signals under dynamic conditions to study fast reactions in chemistry. This study explores the various methods used, such as Flow Method and Stopped Flow Method, to determine reaction rates accurately. Advantages of the Stopped Flow Method over Continuous
0 views • 18 slides
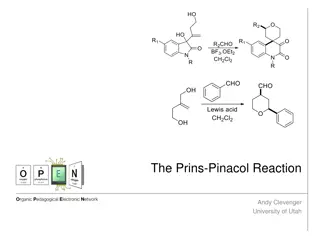
Understanding the Prins-Pinacol Reaction in Organic Chemistry
The Prins-Pinacol reaction involves a two-step process starting with the Prins reaction and followed by the Pinacol rearrangement. This reaction, discovered in 1919 by Hendrick J. Prins, is a crucial transformation in organic chemistry, leading to the formation of important carbonyl compounds. The m
0 views • 14 slides
Understanding Kinetics and Reaction Rates in Chemistry
Kinetics is the study of reaction rates and factors affecting them, such as concentration, temperature, catalysts, and more. Orders of reaction classify reactions based on rate dependency on reactant concentration. Factors like pH, light, and solvents can also impact reaction rates. Half-life and sh
0 views • 18 slides
Understanding Free Radical Polymerization Kinetics
This lecture covers the kinetics of free radical polymerization, including initiation, propagation, termination, and kinetic chain length concepts. It explains the calculation of kinetic chain length and chain-transfer reactions. Key points include the rate equations for initiation, propagation, and
0 views • 11 slides
Introduction to Kinematics and Dynamics of Machines in Mechanical Engineering
Theory of Mechanics delves into motion, time, and forces, with Kinematics focusing on motion analysis without considering external forces. Kinetics, a branch of Theory of Machines, deals with inertia forces resulting from mass and motion. Dynamics combines Kinematics and Kinetics to study motion and
0 views • 14 slides
Factors Affecting Enzyme Activity and Kinetics Experiments
Explore the factors influencing enzyme activity, such as substrate and enzyme concentration, temperature, pH, and inhibitors. Learn how to simulate enzyme kinetics using equipment like popping beads and stopwatches. Analyze results to understand the impact of substrate concentration on reaction rate
0 views • 22 slides
Understanding Chemical Kinetics: Reaction Rates and Mechanisms
Chemical kinetics is a branch of chemistry focused on studying reaction rates and mechanisms. Unlike thermodynamics, which deals with feasibility, kinetics explores the speed at which reactions occur. Factors such as temperature, pressure, and catalysts influence reaction rates. Understanding the ra
3 views • 72 slides
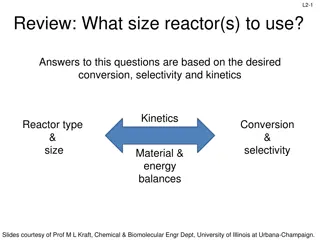
Reactor Sizing: Conversion, Selectivity, and Kinetics Overview
Understanding reactor design involves considerations such as desired conversion, selectivity, and kinetics. Key concepts include rate laws, molar balances, and reactor types. Through molar balance equations and reactor design processes, one can derive essential equations for ideal batch, CSTR, and P
2 views • 20 slides
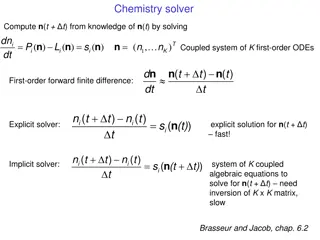
Chemical Kinetics and Numerical Solvers in Chemistry
Explore the principles of chemical kinetics and the use of numerical solvers to compute concentrations over time, considering explicit and implicit methods. Understand stability and positivity requirements in solvers and the importance of characteristic time scales in chemical systems. Dive into a s
0 views • 5 slides
Utilizing a Global Model for Analyzing Reaction Pathways in Plasma Systems
This research focuses on using a kinetic global model framework to identify relevant reactions in chemically complex plasma systems. The framework, KGMf, enables the investigation of macroscopic plasma characteristics by analyzing reaction pathways, sensitivity to reaction rate errors, and dominant
1 views • 6 slides
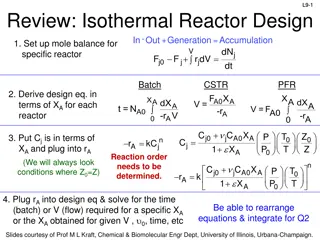
Chemical Reactor Design Review: Mole Balances, Rate Data Analysis, Method of Excess
This content provides a comprehensive review of isothermal reactor design, including setting up mole balances, deriving design equations in terms of conversion, analyzing rate data to determine reaction order and rate constant, and applying the method of excess to evaluate reaction kinetics. Detaile
0 views • 21 slides
Fundamentals of Chemical Kinetics and Reactor Design
Explore the realm of chemical reactions, rate equations, and reactor design in this informative chapter. Understand the factors influencing reaction rates, different types of reactions, rate laws, and experimental determination of reaction rates. Dive into examples illustrating stoichiometry and rat
0 views • 19 slides
Understanding Chemical Reaction Kinetics in Chemcad
Explore the kinetic reactor module in Chemcad for reaction rate specification using VBA. Learn how to determine parameter values and analyze reactions such as steam reforming and water gas shift. Follow step-by-step instructions to run the unit operation, view reactor profiles, and compare reaction
0 views • 9 slides
Overview of Chemical Reactor Design and Operation
Chemical reactor design involves studying the rates and mechanisms of chemical reactions, as well as the design of reactors for these reactions on a commercial scale. This field combines principles from thermodynamics, chemical kinetics, fluid mechanics, mass transfer, heat transfer, and economics t
0 views • 12 slides
Understanding Chemical Reaction Kinetics: From Unimolecular to Three-Body Reactions
Explore the fundamental concepts of chemical reactions, including unimolecular reactions like thermolysis and photolysis, bimolecular reactions, and three-body reactions. Learn about rate constants, reaction mechanisms, and the impact of pressure on reaction rates. Discover how energy transfer, phot
0 views • 9 slides
Understanding the Interface to the Current CCPP: A Simple Model's Perspective
This content explores the interface to the current CCPP from a simple model's viewpoint, focusing on two simplistic schemes - kinetics and chem_solve. It delves into how the MusicBox simple driver model adapts chemical species using temperature and rate constants. Users can control the run sequence
0 views • 9 slides
Understanding Free Energy, Reaction Quotient, and Equilibrium Constant
This educational material delves into the concepts of free energy, reaction quotients, and equilibrium constants in chemical systems. It explains how to determine the direction of a reaction based on Q and K values, elucidates the role of Gibbs free energy in determining spontaneity, and provides ca
0 views • 10 slides
Understanding Chemical Kinetics and Equilibrium in Reactions
Explore the basic concepts of reaction rates, collision theory, activation energy, and energy diagrams in chemical kinetics and equilibrium. Learn how particles must collide with the correct orientation and enough energy to form an unstable activated complex. Discover the role of activation energy i
0 views • 24 slides
Grignard Reaction in Chemistry Lab: Part 1 Overview
The Grignard Reaction Part 1 in Chemistry 318 Fall 2018 involves the preparation of the Grignard reagent, its reaction with CO2, and the isolation of the benzoic acid product. The experiment spans two lab sessions, focusing on safety precautions, pre-lab checks, and upcoming due dates. Students are
0 views • 11 slides
Introduction to Chemical Reaction Engineering (CRE)
Chemical Reaction Engineering (CRE) focuses on studying the rates and mechanisms of chemical reactions, as well as designing reactors for these reactions. The field involves understanding balances in terms of molar flow rates, mole balances, rate laws, stoichiometry, and membrane reactors. Membrane
0 views • 20 slides
Understanding Chemical Kinetics: The Rate of Reaction and Equilibria
Chemical kinetics explores the rate at which chemical reactions occur and the factors influencing them. This tutorial delves into the concepts of reaction rates, equilibrium, collision theory, and the role of concentration in determining reaction rates. By understanding these principles, industries
0 views • 117 slides
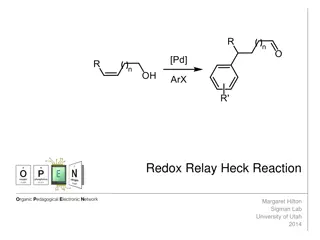
Understanding the Redox-Relay Heck Reaction in Organic Synthesis
The Redox-Relay Heck Reaction is a powerful tool in organic synthesis that allows for the functionalization of olefins with aryl groups. Developed by Sigman and colleagues, this reaction involves a palladium-catalyzed relay controlled by a thermodynamic sink, leading to the formation of aldehydes or
0 views • 6 slides
Understanding Chemical Kinetics: Reaction Rates and Activation Energy
Exploring the fundamental concepts of chemical kinetics, this content delves into reaction rates, collision theory, and activation energy in chemical reactions. It emphasizes the importance of particle collisions, correct orientation, and energy requirements for reactions to occur. Through energy di
0 views • 17 slides
Replication Kinetics in Post-Transplant CMV Infections
This study explores the variation in replication kinetics of Cytomegalovirus (CMV) during post-transplant infections. The aim is to assess the doubling time of CMV and evaluate the rationale behind weekly screening intervals in transplant recipients. Methods include analyzing CMV PCR samples and rev
0 views • 21 slides
Introduction to Chemical Reaction Engineering
Chemical Reaction Engineering (CRE) is crucial for understanding how chemical reactors operate in various processing operations. This field involves reactor design by integrating factors such as thermodynamics, kinetics, fluid mechanics, heat transfer, and economics. CRE aims to effectively design a
0 views • 16 slides
Understanding Kinetics in Chemical Reactions
Kinetics is the study of reaction rates and factors affecting them. Reaction rate is the speed at which a reaction occurs, influenced by factors like concentration, temperature, pH, light, catalysts, and solvents. Reactant concentration determines reaction order, which categorizes reactions as zero-
0 views • 18 slides