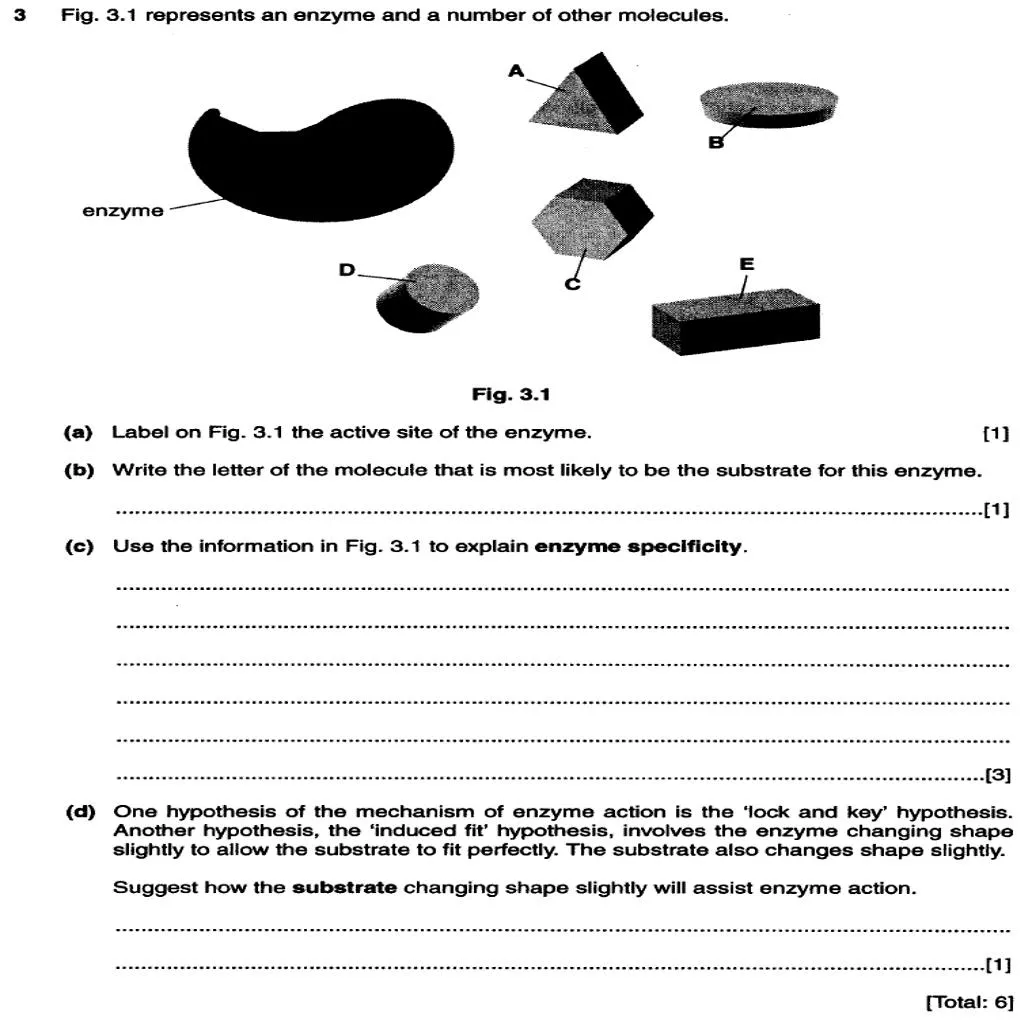
Factors Affecting Enzyme Activity and Kinetics Experiments
Explore the factors influencing enzyme activity, such as substrate and enzyme concentration, temperature, pH, and inhibitors. Learn how to simulate enzyme kinetics using equipment like popping beads and stopwatches. Analyze results to understand the impact of substrate concentration on reaction rates. Discover the relationship between substrate concentration and reaction rate through graphs and exam questions.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Factors Affecting Enzyme Activity
Factors Affecting Enzyme Activity O Concentration of the substrate O Concentration of the enzyme O Temperature O pH O Presence of inhibitors
Simulating Enzyme Kinetics O Equipment O Popping beads O Trays O Stopwatches O Work in groups of three A is the Enzyme B passes the beads to the enzyme at a set rate (collision rate) C manages the stop clock.
Results Time Time interval interval between between passing to passing to enzyme enzyme (s) (s) 10 10 Substrate Substrate Concentration Concentration (Beads (Beads passed/minute) passed/minute) Reaction Rate Reaction Rate (Beads (Beads joined /minute) joined /minute) 6 6 10 10 12 12 15 15 20 20 30 30 60 60 6 6 5 5 4 4 3 3 2 2 1 1
Increasing the substrate concentration O As concentration of substrate increases, collisions between enzyme and substrate molecules occur more often. O More enzyme-substrate complexes form, so more product is formed. O The reaction rate increases Page 132-135
The effect of substrate concentration on the rate of an enzyme controlled reaction 7 7 -- -- 6 6 -- -- Why has the rate levelled out? What is limiting any further increase in the rate of reaction ? Rate of reaction (au) 5 5 -- -- 4 4 -- -- 3 3 -- -- Here the increase in rate and substrate conc are proportional 2 2 -- -- 1 1 -- -- | | | | | | | | | | 1 2 3 4 5 1 2 3 4 5 Substrate conc (mol/dm ) Exam question. Exam question. Describe the relationship between the substrate concentration and the rate of the reaction. Look for overall trends Refer to points on the graph Do not generalise
Increasing the enzyme concentration O As the enzyme concentration increases, more active sites become available. O More enzyme-substrate complexes form (and so more products are produced). The rate of reaction increases. Sketch a graph to show the relationship between enzyme Sketch a graph to show the relationship between enzyme concentration and the rate of reaction. concentration and the rate of reaction. What happens if What happens if the substrate the substrate concentration increases further? concentration increases further? Draw another line to show what the reaction would look like if Draw another line to show what the reaction would look like if there was double the substrate concentration there was double the substrate concentration 1. 1. 2. 2. 3. 3.
Enzymes and temperature O Kinetic energy and collision theory O Heat, vibration, breaking bonds and denaturation Give a definition of denaturation Give a definition of denaturation Describe how heating can lead to denaturation in enzyme Describe how heating can lead to denaturation in enzyme structure structure Textbook p128-9
Optimum temperatures Refer to the graph to explain the effect of temperature on enzymes. Include: Include: Kinetic energy, Collision theory , enzyme/substrate complexes, Kinetic energy, Collision theory , enzyme/substrate complexes, denaturation denaturation Textbook p128-9
Precise terminology Precise terminology Formation of enzyme substrate complexes Increase in the kinetic energy of the substrate and enzyme molecules High temperatures disrupt the tertiary structure of the enzyme by breaking the tertiary bonds
Enzymes and pH O What is pH? O pH and bonds (interfere with hydrogen bonds and ionic bonds-tertiary structure affected) Textbook p130-131
pH and active site Importance in terms of induced-fit hypothesis Increasing H ions, alters charges around the active site Textbook p130-131
O Varies depending on enzyme (e.g. where found) O At optimum, concentration of H ions in solution gives the tertiary structure the best overall shape. O Range is generally fairly narrow Optimum pH Buffer solutions at set pH values are used to carry out enzyme reactions. The rate can then be calculated and the optimum found. Denaturation will only occur in extreme changes of pH away from the optimum. The further from the optimum the faster the rate of denaturation will be. Textbook p130-131
Inhibitors An enzyme inhibitor is any substance or An enzyme inhibitor is any substance or molecule that slows down the rate of an molecule that slows down the rate of an enzyme enzyme- -controlled reaction by affecting the controlled reaction by affecting the enzyme molecule in some way enzyme molecule in some way. . O Competitive Competitive Similar in shape to substrate O Non competitive Non competitive Do not compete with substrate molecules for a place in the active site O Permanent Permanent Most competitive inhibitors are not permanent (action is reversible). Many non competitive inhibitors bind permanently to enzyme molecules
Enzyme inhibitors forward_arrow_colour
S Normal enzyme substrate binding: E Irreversible inhibition O The inhibitor binds permanently with the enzyme and changes the shape of the active site preventing substrate binding. O e.g. heavy metal ions mercury, lead S Substrate no longer fits into active site and effect is irreversible E I I
Reversible inhibition e.g. Leeches produce hirudin which inhibits the production of a blood clotting protein e.g. cyanide inhibits an enzyme involved in respiration O Non-competitive O Competitive S S I I E E E Inhibitor binds at another site (allosteric site) on the enzyme but changes the shape of the active site Substrate cannot bind, even if its concentration is increased Effect is however reversible if inhibitor is removed shape of active site is restored I If inhibitor occupies the active site and substrate can no longer bind Increasing concentration of substrate reduces effect of inhibitor
With a competitive inhibitor With a non competitive inhibitor Rate Of reaction Rate Of reaction Normalrate Inhibited rate Substrate concentration Substrate concentration Decide which graph represents the effect on the rate of an enzyme controlled reaction when exposed to: a competitive inhibitor a non competitive inhibitor. Each reaction is carried out with a fixed quantity of inhibitor.
End-product inhibition O The product of the reaction which is being catalysed acts an inhibitor of the enzyme involved substrate products 1. Substrate binds at active site 2. Reaction is catalysed 3. Product inhibits enzyme (usually non- competitive)
True or false? True True Well Well Well Well False False False False O An enzyme is a biological catalyst O An enzyme will work faster at 60 C O An enzyme will be denatured at 100 C O Enzymes break things down O Enzymes are denatured if chilled, which is why a fridge preserves food O There will be as much enzyme left at the end of a reaction, as there was at the beginning O Blank-ase breaks down or interacts with blank True True True True