Conductometry: An Overview of Measurement and Applications
Conductometry involves measuring the conductivity of a solution to determine its properties. This method includes factors affecting conductivity, conductometric titration, recent developments, and application examples. The process involves using electrodes, primary standard solutions, Wheatstone bri
9 views • 24 slides
Understanding Chemical Quantities: The Mole and Molar Mass
Explore the concept of chemical quantities through the mole and molar mass. Learn how to measure substances, calculate moles, find molar masses of compounds, and solve related problems in this informative chapter. Discover the significance of Avogadro's number, representative particles, and more in
7 views • 41 slides
Understanding Chemical Potential and Phase Equilibria in Solution Thermodynamics
The chemical potential and phase equilibria in solution thermodynamics are crucial concepts for understanding the behavior of mixtures at varying compositions and conditions. By investigating the fundamental property relation, partial molar properties, and the role of Gibbs energy, we can grasp how
5 views • 25 slides
Understanding Polymers and Their Properties
Polymers are long chains of repeating monomers, with both natural and synthetic varieties. Natural polymers include silk, cellulose, and DNA, while synthetic ones encompass plastics, fibers, and elastomers. The properties of polymers, such as molar mass and monomer structure, determine their functio
0 views • 15 slides
Understanding Kinetic Theory of Gases: Key Concepts and Equations
Exploring the kinetic theory of gases, this content covers essential concepts such as ideal gas behavior, molar mass, the equation of state, and isobaric/isothermal processes. Discover the relationship between pressure, volume, and temperature in gases, along with practical examples and calculations
0 views • 49 slides
Stoichiometry Test Review Sheet
A comprehensive review sheet covering key concepts in stoichiometry, including molar ratios, coefficients in chemical equations, determining actual yield in reactions, identifying limiting reactants, calculating percentage yield, and understanding mole ratios in chemical reactions. The review provid
0 views • 11 slides
Understanding Mole Calculations in Chemistry
Explore various mole calculations in chemistry such as determining mass from moles, moles from mass, and comparing particles in different substances. Learn how to calculate the mass of substances, the number of particles, and perform calculations using balanced equations. Dive into concepts like mol
0 views • 49 slides
Understanding Percent Composition and Empirical Formulas in Chemistry
The Law of Definite Proportions governs the composition of compounds based on molar masses, allowing us to calculate percentage compositions of elements within a compound. Through examples involving various compounds like Fe3C, sulfur dioxide, ammonium nitrate, glucose, and acetic acid, we explore t
3 views • 7 slides
Understanding Activity and Fugacity in Gases
Fugacity of real gases can be measured, defining standard states in terms of fugacity. The relation between fugacity, activity, and molar concentration is explored, highlighting the ideal behavior of gases at standard states. Activity coefficients and deviations from ideal gas behavior are also disc
0 views • 30 slides
Understanding Electrolytes in Chemical Solutions and Their Importance in Body Fluid Balance
Chemical compounds in solution can remain intact or dissociate into ions, affecting their electrical charge. Electrolytes like sodium chloride play a crucial role in body fluid balance and acid-base regulation. Electrolyte preparations are used to treat imbalances, with milliequivalents and molar co
0 views • 32 slides
Understanding Electrolytes in Chemical Solutions
The content discusses the concept of electrolytes in chemical compounds, exploring how they can remain intact or dissociate into ions in solution. It explains the roles of electrolytes in body fluids, their movement in the presence of electrodes, and the use of electrolyte preparations in clinical p
3 views • 25 slides
Preparation of Solution from Solid: Practical General Chemistry Techniques
Solutions are homogeneous mixtures where a solute is dissolved in a solvent. Known concentration solutions can be prepared using solid substances through careful techniques. This process involves weighing the solid, dissolving it in a beaker, transferring to a volumetric flask, and finalizing the so
0 views • 12 slides
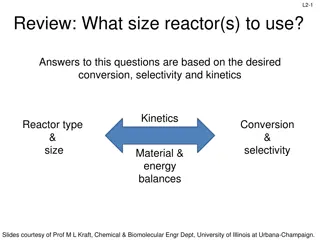
Reactor Sizing: Conversion, Selectivity, and Kinetics Overview
Understanding reactor design involves considerations such as desired conversion, selectivity, and kinetics. Key concepts include rate laws, molar balances, and reactor types. Through molar balance equations and reactor design processes, one can derive essential equations for ideal batch, CSTR, and P
2 views • 20 slides
Understanding Real Gas Behavior in Chemistry
Real gases deviate from ideal gas behavior due to factors like finite volume of gas particles and intermolecular attractions. At high pressures and low temperatures, real gases don't follow ideal gas laws, impacting their molar volume and pressure calculations. This deviation is crucial in analyzing
1 views • 11 slides
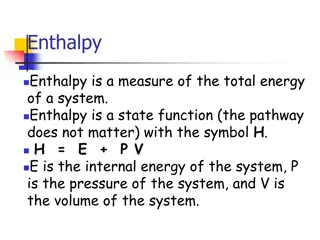
Understanding Enthalpy and Heat Capacity in Chemistry
Enthalpy is a measure of total energy in a system, represented as H = E + P.V. Heat at constant pressure relates to enthalpy changes. Calorimetry and heat capacity help measure and understand heat in chemical reactions. Specific heat capacity and molar heat capacity play key roles in determining ene
0 views • 16 slides
Understanding Concentration of Solutions in Physiology
Concentration of solutions is crucial in understanding the properties of substances in Physiology. This involves concepts like percentage solutions and molar solutions, where the amount of solute is measured in grams or moles relative to the volume of the solution. Percentage solutions are commonly
0 views • 8 slides
Dental Anatomy and Nomenclature by M.E. Mermigas, DDS
Detailed images and descriptions providing insight into dental anatomy, nomenclature, deciduous and permanent teeth, crown and root structure, dentin composition, pulp chamber and canal features, tooth crown characteristics, and individual tooth types (incisor, canine, premolar, molar).
0 views • 40 slides
Introduction to Chemical Reaction Engineering (CRE)
Chemical Reaction Engineering (CRE) focuses on studying the rates and mechanisms of chemical reactions, as well as designing reactors for these reactions. The field involves understanding balances in terms of molar flow rates, mole balances, rate laws, stoichiometry, and membrane reactors. Membrane
0 views • 20 slides
Understanding Percentage Composition in Chemistry
Percentage composition is crucial in determining the elemental composition of compounds. This lesson explains how to calculate percentage composition using molar masses, illustrated with examples like copper sulfide. It also delves into hydrated salts and their significance in chemistry. Students wi
0 views • 22 slides
Understanding Limiting Reactants in Chemistry
In chemistry, the limiting reactant is crucial as it determines the amount of product that can be formed in a reaction. By identifying and solving for the limiting reactant, you can find the maximum amount of product that can be obtained. This process involves understanding stoichiometry, calculatin
0 views • 16 slides
Understanding Conductivity in Saline Water Solutions
Study the relationship between dissolved ions and conductivity in saline water solutions to determine molar conductivity. Learn about conductive solutions, advantages/disadvantages, and factors influencing conductivity. Engage in hands-on experiments using SensorLab conductivity sensors to investiga
0 views • 22 slides
Understanding Toxicity Through Stoichiometry and Molar Mass
Delve into the world of toxicity analysis by comparing the amounts of different substances using moles and molar mass. Explore the safety of sweeteners and learn how to utilize these concepts to assess toxicity levels. Engage in thought-provoking discussions and activities to deepen your understandi
0 views • 10 slides
Understanding Molar Mass and Avogadro's Number in Chemistry
Explore the concept of molar mass and Avogadro's number in chemistry through lessons on translating numbers into scientific notation, understanding moles, and finding molar mass on the periodic table. Discuss the relationship between mass and moles, differentiate between different quantities of a su
1 views • 11 slides
Understanding the Mole Concept in Chemistry
Delve into the world of chemistry with the Mole Concept, exploring molar mass, Avogadro's number, representative particles, and more. Learn how to determine molar mass for compounds and grasp the significance of a mole in chemical calculations.
0 views • 27 slides
Understanding Unit Conversion and Mole Concept in Chemistry
Explore the concepts of unit conversion and the mole in chemistry, including how to convert between different units, relate mass to atoms and molecules, calculate molar mass, and perform conversions involving substances like chalk and sodium hydroxide. Discover the importance of dimensional analysis
0 views • 12 slides
Understanding Thermal Physics and Gas Laws in Physics Class
Explore the concepts of thermal physics and gas laws in today's physics class. Learn about the transfer of heat, the mole concept, atomic and molar masses, pressure, standard pressure and temperature (STP), and ideal gases. Delve into calculations involving sample materials like copper and water to
0 views • 20 slides
Understanding Moles and Molar Quantities in Chemistry
Chemistry involves understanding atomic structure, chemical equations, and measurement units like moles. Avogadro's number, elemental composition, and compound formulas play crucial roles in calculating and using substances in chemical reactions. Learn about the significance of mass numbers, moles,
0 views • 25 slides
Understanding the Kinetic Theory of Gases and Ideal Gas Model
Explore the Kinetic Theory of Gases, the Molecular Model of Ideal Gases, and concepts like pressure, temperature, and heat capacity. Understand the fundamental properties of gases, including the distribution of molecular speeds and equations that describe gas behavior. Learn about the mole concept,
0 views • 23 slides
Understanding Stoichiometry and The Mole Concept in Chemistry
Explore the fundamentals of stoichiometry and the mole concept in chemistry, including conversions between moles and particles, molar mass calculations, and gram mole conversions. Learn how to determine the number and kinds of atoms in chemical formulas and understand the significance of Avogadro's
0 views • 12 slides
Understanding the Concept of Molar Mass in Chemistry
Explore the concept of molar mass in chemistry, including the definition of the mole, Avogadro's number, calculations for molar mass of elements and compounds, and examples of determining molar mass. Discover how to find the molar mass of various compounds through practical examples.
0 views • 39 slides
Understanding Moles in Chemistry
Matter is composed of various particles, and chemists use the concept of moles as a unit of measure to quantify the number of particles in a substance. One mole is equal to 6.02 x 10^23 representative particles of a substance, known as Avogadro's number. Moles are versatile and applicable to differe
0 views • 25 slides
Understanding Molar Conductivity of Strong Electrolytes
Strong electrolytes are materials highly dissociated in water, leading to conducting solutions with high molar conductivity. This article delves into the concept, calculation methods, and experimental procedures for determining the molar conductivity of strong electrolytes using examples of NaCl, KC
0 views • 5 slides
Understanding Standard Molar Enthalpies of Formation
Formation reactions involve substances being created from elements in their standard states, with the enthalpy change known as the standard molar enthalpy of formation (Hf). This enthalpy represents the energy released or absorbed when one mole of a compound is formed from its elements in their stan
0 views • 13 slides
Understanding Partial Molar Quantities and Chemical Potential
Exploring partial molar quantities and chemical potential in thermodynamics helps us understand how system variables change with composition alterations. Through concepts like partial molar volumes and Gibbs free energy, we can delve into the intricate dynamics of thermodynamic systems and their beh
0 views • 23 slides
Understanding Molar Mass and Conversions in Chemistry
Explore the concept of molar mass, converting between grams and moles, determining mass and moles of elements, and calculating the number of atoms in samples. Practice exercises help reinforce learning in this comprehensive chemistry topic.
0 views • 15 slides
Physical Chemistry I - Semester 2 Outlines and Practice Questions
Dive into Physical Chemistry I with this detailed outline covering topics such as molar mass of gas, gas mixtures, and ideal gas equations. Practice questions on gas pressure, volume, and temperature relationships are included for self-assessment. Learn how to calculate molar mass, determine gas den
0 views • 27 slides
Understanding Molar Mass and Conversions in Chemistry
Explore the concept of molar mass, moles of atoms, and conversions between mass and moles in chemistry through an engaging lesson on toxins, stoichiometry, solution chemistry, and acids and bases. Learn how to calculate molar mass, describe the magnitude of a mole of a substance, and conduct simple
0 views • 10 slides
Understanding Chemical Quantities: The Mole Concept and Molar Mass
Chemists use the mole concept to relate mass and the number of atoms in chemical reactions. Avogadro's number, molar mass, stoichiometry, and energy changes in reactions are key concepts explored in this chapter. The mole is a vital unit in chemistry, enabling scientists to quantify substances and m
0 views • 77 slides
Understanding Heat and Temperature Changes in Chemistry
Heat and temperature changes in chemistry are crucial concepts to comprehend. Heat capacity, molar heat capacity, and specific heat capacity play significant roles in determining temperature changes when heat energy is added or removed. Different substances have varying abilities to absorb heat, aff
0 views • 17 slides
Understanding Heat Transfer in Phase Changes of Water
Water molecules exhibit different behaviors in the liquid and gaseous states due to varying attractions between molecules. To change liquid water to a gas, energy must be added to overcome intermolecular forces, making this process endothermic. The heat absorbed during melting is equal to the heat r
0 views • 22 slides