Understanding Unit Conversion and Mole Concept in Chemistry
Explore the concepts of unit conversion and the mole in chemistry, including how to convert between different units, relate mass to atoms and molecules, calculate molar mass, and perform conversions involving substances like chalk and sodium hydroxide. Discover the importance of dimensional analysis and the molar mass in chemistry calculations.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
How small is an atom? How many moles of chalk does it take to write your name? _____ g chalk (CaCO3) I mole ________atoms __________ g CaCO3 1 mole
: Unit conversions (dimensional analysis) allows us to compare two separate units of measure by using a conversion factor. Example: 2 dozen eggs = ? Eggs 12 eggs = __X___ 1 doz. 2 doz. here s the math: 2 dozen x 12eggs = 24 eggs dozen [12 eggs/dozen] is the conversion factor that lets us compare eggs & dozen
Unit Conversion & dimensional analysis Set up to cancel units: 2 dozen x 12eggs = 24 eggs dozen This set up allows us to make many comparisons in one calculation: Ex: Convert 12 days to seconds: 12 days x 24 hrs. X 60 min. X 60 sec = 1036800 sec. 1 day 1 hr. 1 min
Unit conversion and dimensional analysis: Determine the final label by crossing out the units that cancel: 1) mi x hr x min x m x cm = hr min s mi m 1) mi x hr x min x m x cm = cm hr min s mi m s
Unit Conversions: Convert 3.0 centimeters to feet Strategy: cm inches feet 3.0 cm x 1 in x 1 ft = 2.54 cm 12 in
: The unit mole now allows us to relate mass to atoms and molecules. This is important since atoms and molecules are so small , we can use the unit mole to obtain tangible measurements. Molar mass describes how many grams in one mole of an element or compound. It is a conversion factor between grams and moles.
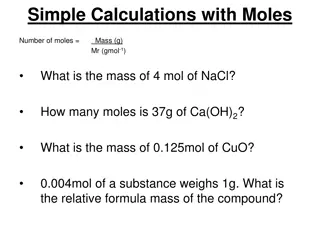
: 1. Molar Mass describes how many grams in one mole of an element or compound. It is determined just like formula mass. We add the mass of all the contributing elements. This information is found on the periodic table. 2. When converting mass to moles we divide by the molar mass. 3. When converting moles to mass we multiply by the molar mass.
Calculate the molar mass of sodium hydroxide (NaOH) Na = 23.0g O = 16.0g H = 1.0 g 40.0 g/mole Convert 226g of NaOH to moles: Convert 1.4 moles of NaOH to grams: Convert 28.0 grams of NaOH to moles:
Grams Moles Moles Grams Moles Find the number of moles in 92.2 g iron Grams Calculate the mass, in grams, of 0.250 moles of sodium. Atoms Calculate the number of atoms in 3.2 moles of carbon Atoms Calculate the number of atoms in 2.5 grams of silver.