Understanding Molar Mass and Conversions in Chemistry
Explore the concept of molar mass, converting between grams and moles, determining mass and moles of elements, and calculating the number of atoms in samples. Practice exercises help reinforce learning in this comprehensive chemistry topic.
Uploaded on Oct 09, 2024 | 0 Views
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
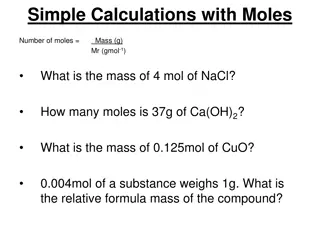
Learning Goals 0Convert between grams and moles. 0Find molar mass of an element.
The Mass of a Mole 0Do 12 large nails have the same mass as 12 small nails?
The Mass of a Mole 0Does 1 mol of sulfur have the same mass as 1 mol of carbon?
The Mass of a Mole 0Molar mass is the mass in grams of one mole of any pure substance. 0The molar mass of any element is numerically equivalent to its atomic mass and has the units g/mol.
The Mass of a Mole = 55.845 g
Using Molar Mass 0Converting from moles to mass:
Using Molar Mass 0What is the mass of 3.00 moles of copper?
Using Molar Mass 0Converting from mass to moles:
Practice 0Determine the number of moles in each of the following: 025.5 g Ag 0300.0 g S
Practice 0Determine the mass of each of the following: 03.57 mol of Al 042.6 mol of Si
Using Molar Mass 0How many atoms are in each of the following samples? 055.2 g Li 00.230 g Pb 011.5 g Hg
Using Molar Mass 0What is the mass in grams of each of the following? 06.02 x 1024 atoms Bi 01.00 x 1024 atoms Mn 03.40 x 1022 atoms He