Understanding Moles and Molar Quantities in Chemistry
Chemistry involves understanding atomic structure, chemical equations, and measurement units like moles. Avogadro's number, elemental composition, and compound formulas play crucial roles in calculating and using substances in chemical reactions. Learn about the significance of mass numbers, moles, and elements in chemical formulas.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
Number of protons, which is unique to that element is atomic number The mass number of an element - the sum of protons plus neutrons in the nucleus of an atom
In chemistry, we calculate and measure the amounts of substances to use in lab Like in everyday life Chemical reactions occur everywhere Simple or complex All can be written with chemical equations Reactants and products
In grocery store we use certain scale for food Dozen (eggs), case (soda), gross (pencils) ream (paper) Example: dozen eggs = 12 eggs
Mole In chemistry, particles such as atoms, molecules, and ions are counted by the mole, which contains 6.02 x 1023items. This value, known as Avogadro s number, is a very big number because atoms are so small that it takes an extremely large number of atoms to provide a sufficient amount to weigh and use in chemical reactions. Avogadro s number is named for Amedeo Avogadro 1776 1856, an Italian physicist.
Avogadros number tells us that one mole of a compound contains 6.02 x1023 of the particular type of particles that make up that compound. 1 mole of carbon contains 6.02 x 1023carbon atoms; 1 mole of aluminum contains 6.02 x 1023aluminum atoms; 1 mole of sulfur contains 6.02 x 1023sulfur atoms
1 mole of an element = 6.02 x 1023 atoms of that element
One mole of a molecular compound contains Avogadro s number of molecules Example: 1 mole of CO2 contains 6.02 x 1023molecules of CO2.
Moles of Elements in a Chemical Formula Subscripts indicate the number of atoms of each type of element in the compound Example: aspirin (C9H8O4) has 9 carbon atoms, 8 hydrogen atoms and 4 oxygen atoms Also tells the number of moles of each element in 1 mole of aspirin 9 moles of C atoms, 8 moles of H atoms and 4 moles of O atoms
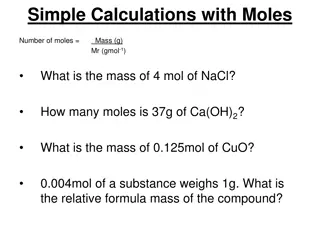
Molar Mass and Calculations Single atom or molecule is too small to weigh It takes a huge number of atoms or molecules to make enough of a substance for you to see Molar mass quantity in grams that equals the atomic mass of that element Example: carbon has atomic mass of 12.01 1 mole of carbon atoms has a mass of 12.01 g To obtain 1 mole of carbon atoms we must weigh out 12.01 g of carbon
The molar mass of an element is useful to convert moles of an element to grams, or grams to moles We can calculate molar mass of a compound
Problem 1 Li2CO3 Obtain the molar mass of the compound
Solution 1 1. obtain the molar mass of each element: Li= 6.941 g/mol C= 12.01 g/mol O= 16 g/mol 2. Multiply each molar mass by the number of moles (subscript) in the formula 3. Calculate the molar mass by adding the masses of elements 4. Solution: 73.9 g/mol
Problem 2 Determine the molar mass of the elements: Pt, U, Mg, Mn, Fe, Zn C, O, N, H, S Bi, Po, Xe, Rn, Na
Solution 2 Pt (195 g/mol) U (238 g/mol), Mg (24.3 g/mol), Mn (55 g/mol), Fe (56 g/mol), Zn (65.4 g/mol) C (12 g/mol), O (16 g/mol), N (14 g/mol), H (1 g/mol), S (32 g/mol) Bi (209 g/mol), Po (210 g/mol), Xe (131.3 g/mol), Rn (220 g/mol), Na (23 g/mol)
Problem 3 Determine the molar mass of molecules: Ag3, N2, O2, H2 Mg2, S2,
Solution 3 Ag2(2 x 107.9 g/mol= 215.8 g/mol), N2(2 x 14 g/mol= 28 g/mol), O2(2 x 16 g/mol= 32 g/mol), H2(2 x 1 g/mol= 2 g/mol) Mg2(2 x 24.3 g/mol= 48.6 g/mol), S2(2 x 32 g/mol= 64 g/mol)
Problem 4 Calculate the molar masses of compounds: AgNO3, NaCl, AgCl, NaNO3 C13H18O2(Ibuprofen) PtCl2(NH3)2(Cisplatin) C6H12O6(Glucose)
Solution 4 Single work! AgNO3 = 107.9 + 14 + 3 x 16= 170 g/mol NaCl= 23 + 35.4= 58.4 g/mol AgCl= 107.9 + 35.4= 143.3 g/mol NaNO3= 23 + 14 + 3 x 16= 85 g/mol C13H18O2= 13 x 12 + 18 x 1 +2 x 16= 206 g/mol PtCl2(NH3)2= 195 + 2 x 35.4 + (2 x 14 + 6 x 1)= 299.8 g/mol C6H12O6= 6 x 13 + 12 x 1 + 6 x 16= 186 g/mol
Conclusions Calculations of molar mass!
Assignments Calculate the molar mass of nickel (II) hexahydrate (NiSO4 6H2O) Calculate the molar mass of the hydrates formula unit NiSO4 Calculate the molar mass of PO4H2(CH2)12CH3 Calculate the molar mass of C17H29COOH (Linoleic acid) Calculate the molar mass of C H NO (Morphine)