Europe EV battery management system market size 2029
the European EV batteries market is mainly segmented into wire bonding and laser bonding. In 2022, the wire bonding segment is expected to account for a largest share of the European EV batteries market. The large market share of this segment is mainly attributed to its ability of fast and fully aut
0 views • 5 slides
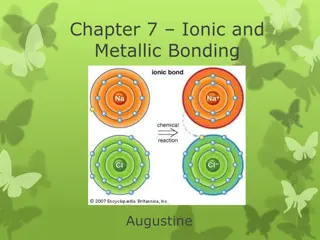
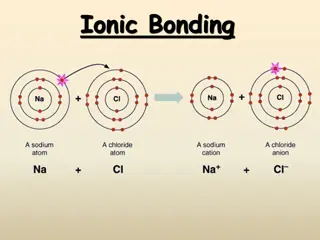
Ionic and Metallic Bonding: Valence Electrons, Octet Rule, and Ion Formation
Explore the essential concepts of ionic and metallic bonding, focusing on valence electrons, electron dot structures, the octet rule, cations, anions, and ion formation. Discover how atoms achieve stability through electron transfer, and learn to write electron configurations for various ions.
9 views • 52 slides
Ionic and Metallic Bonding in Chemistry
Explore the concepts of ionic and metallic bonding in chemistry through discussions on valence electrons, electron dot structures, the octet rule, cations, anions, and more. Dive into the world of ions and electron configurations to understand how atoms achieve stability through the gain or loss of
3 views • 62 slides
Ionic and Covalent Bonding in Chemistry
Ionic bonding involves the transfer of electrons between a metal and a non-metal to form a giant lattice structure, like in sodium chloride and lithium oxide. Covalent bonding, on the other hand, occurs between non-metals, resulting in giant covalent structures or simple molecules. Examples such as
4 views • 79 slides
Philosophy of Thales: The Ancient Wisdom of the Ionic School
Thales of Miletus, a key figure in the Ionic School of philosophy, is considered the father of philosophy. He believed that water was the principle of all things and made significant contributions to mathematics and astronomy. Thales's wisdom and engineering feats, such as diverting the river Halys,
0 views • 18 slides
Trauma Bonding in Trafficked Youth
Trauma bonding is an emotional attachment that develops between abusers and victims, often seen in youth who have been trafficked. This bond can make it difficult for victims to break free from exploitation. Understanding trauma and its impact on the brain is crucial in supporting these individuals
0 views • 21 slides
Ionic and Metallic Bonding in Chemistry
Explore the concepts of ions, electron dot structures, the octet rule, cations, and anions in Chapter 7. Learn how elements achieve stability through electron configurations, and practice writing electron dot structures and naming ions. Understand the differences between cations and anions and how t
2 views • 52 slides
Covalent Bonding in Chemistry
Explore the concept of covalent bonding in chemistry, where atoms share electrons through orbital overlap to form stable molecules. Learn about why covalent bonds exist, how bond length affects the stability of a molecule, the model for covalent bonding, Lewis structures, and the characteristics of
0 views • 12 slides
Borane Cluster Structures and Styx Rule
Boranes are cluster compounds of boron and hydrogen with unique structural arrangements. The Skeletal Electron Count and Styx Rule help in determining the bonding features and types of bonds present in borane compounds. Various borane structures such as Closo, Nido, and Arachno exhibit different bon
1 views • 11 slides
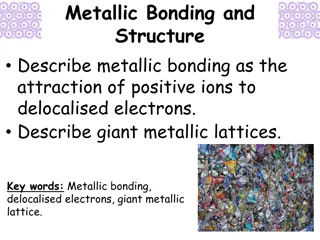
Metallic Bonding and Giant Metallic Lattices
Metallic bonding involves the attraction of positive metal ions to delocalized electrons, forming giant metallic lattices. In this structure, positive metal ions occupy fixed positions while electrons move freely throughout. This bonding is different from covalent bonding as it is delocalized, leadi
1 views • 19 slides
Chemistry Concepts: Valence Electrons, Ion Charges, and Ionic Compounds
Explore various key concepts in chemistry such as valence electrons in magnesium, Lewis Dot structure for silicon, charges on ions like strontium, formation of ions to achieve noble-gas electron configuration, elements forming ions with specific charges, and the octet rule. Learn about the character
1 views • 48 slides
Ionic Bonding and Lattice Energy
Explore the world of ionic bonding through images and explanations. Learn how electrons are transferred to form ions, the arrangement of ions in a crystal lattice, and the concept of lattice energy in ionic compounds. Discover the formation of formula units, examples of bond pairs, and the significa
1 views • 18 slides
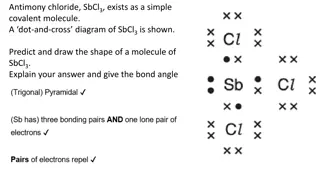
Chemical Bonding Concepts and Structures Explanation
Explore the concepts of chemical bonding through dot-and-cross diagrams for molecules like Antimony Chloride (SbCl3) and Boron Tribromide, along with explanations on ionic lattice structures, covalent bonds, and electrical conductivity in substances like Aluminium Fluoride (AlF3). Understand the sha
0 views • 9 slides
Rejuvenate with Ionic Foot Detox in New Jersey
Experience the benefits of ionic foot detox at Zen Health & Wellness Center in New Jersey. Detoxify, relax, and rejuvenate for improved overall well-being. Book your session today.\nFor more information:\nCall us: 8564620579\nMail us: ZENHEALTHANDWEL
4 views • 8 slides
Valence Electrons and Ionic Charges in Elemental Bonding
Valence electrons play a crucial role in the formation of ions as elements combine. Nonmetals gain electrons to become negatively charged ions, while metals lose electrons to become positively charged ions. This process leads to the creation of electrically attractive elements open for bonding. The
1 views • 17 slides
Bonding in Chemistry
Delve into the world of chemical bonding through ionic, covalent, and metallic bonds. Explore how elements form bonds, from the attraction between sodium and chloride ions to the sharing of electrons in covalent bonds. Witness the formation of compounds like sodium chloride and magnesium oxide, unde
1 views • 12 slides
Chemical Bonding and Stability in Atoms
Explore the significance of chemical bonds in providing stability to atoms through ionic and covalent bonding mechanisms. Learn about valence electrons, types of bonds, and why atoms form bonds for enhanced stability.
0 views • 16 slides
Chemical Bonding and Compound Formulas: Understanding Ionic vs. Covalent Bonds
Explore the differences between ionic and covalent bonds, learn about ionic compounds held by electromagnetic attractions, understand molecular compounds with shared electrons, and grasp the naming conventions for ions. Discover how molecular formulas and formula units represent atoms in compounds.
1 views • 56 slides
Comprehensive Overview of Lewis Model in Chemical Bonding
Examining the coverage of Exam 3 and final exam topics including PV=nRT problems, qualitative features of kinetic theory of gases, atomic dimensions, spdf song, ionic compound predictions, Lewis octet structures, formal charges, VSEPR theory predictions, oxyanion examples of resonance, and the signi
0 views • 19 slides
Interactions of Planar Organic Radicals: Stacking and Bonding
Examination of the stacking interactions and bonding in planar organic radicals reveals a variety of non-covalent and weak covalent interactions such as hydrogen bonding, halogen bonding, and pancake bonding. This study highlights the significance of multicentric two-electron bonding and explores th
0 views • 29 slides
Chemical Bonding: Valency, Formulas, and Reactions
Explore the world of chemical bonding with this unit covering valencies, chemical formulas, ionic vs. covalent bonds, and exothermic vs. endothermic reactions. Learn to predict element combinations, create molecular formulas, and differentiate between various bond types. Jigsaw diagrams demonstrate
1 views • 46 slides
Ions, Ionic Bonds, and Ionic Compounds
Ions are charged particles formed by gaining or losing electrons, leading to the formation of ionic bonds between positively charged cations and negatively charged anions. The octet rule guides electron configurations, and the periodic table helps predict ion formation. Ionic bonding involves electr
0 views • 13 slides
Chemistry Revision Mind Map Unit 1 Summary
Exploring Unit 1 of Chemistry revision, we delve into bonding of the first 20 elements, trends in the periodic table, structure and bonding concepts, and oxidation and reduction reactions. Topics covered include melting points, boiling points, covalent radius, ionization energy, types of bonding, in
0 views • 4 slides
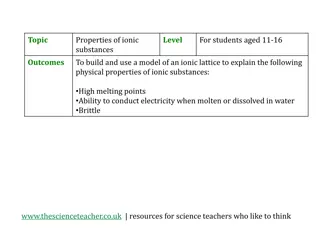
Physical Properties of Ionic Substances through Model Building
Engage students aged 11-16 in an interactive activity to understand the physical properties of ionic substances such as high melting points, ability to conduct electricity, and brittleness. By building a model of an ionic lattice for sodium chloride and explaining how the structure relates to these
0 views • 5 slides
Small Contractors Bonding and Business Planning Initiative
Small Contractors Initiative focuses on providing bonding and access to capital for small businesses. The course covers topics such as bonding basics, business planning, financial management, working capital, and contracting opportunities. Participants will learn the importance of bonds, the history
0 views • 35 slides
Small Contractors Bonding and Access to Capital Initiative
This session focuses on the bonding application process for small contractors, covering key principles, types of bonds, underwriting criteria, and factors that make a contractor a good risk. It addresses why contractors fail, the importance of reputation and financial information, and the roles of b
0 views • 30 slides
Chemical Bonding and Atomic Properties
Explore the formation of ionic and covalent bonds, electron configurations of ions, and molecular geometry. Learn about ionic compound formation, atomic properties like effective nuclear charge, atomic size, ionization energy, and electron affinity. Discover the essential concepts of cations formati
1 views • 73 slides
Weak Interactions and Hydrogen Bonding in Molecular Forces
Exploring van der Waals forces, hydrogen bonding, and weak interactions in intermolecular forces and surface interactions. Understanding interactions between backbone peptide groups and orientation dependence of hydrogen bonding through dispersion forces and repulsive potentials.
0 views • 22 slides
Electrolytes and Ionic Equilibrium in Chemistry
Electrolytes play a crucial role in conducting electricity through ionization in aqueous solutions, with examples of strong and weak electrolytes explained. The concept of ionic equilibrium, degree of dissociation, and distinction between non-electrolytes are also covered in this comprehensive overv
0 views • 21 slides
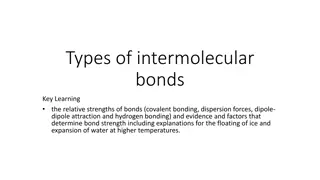
Intermolecular Bonds and Relative Strengths
Understanding the types of intermolecular bonds - covalent bonding, dispersion forces, dipole-dipole attraction, and hydrogen bonding, along with their relative strengths and factors determining bond strength. Learn about permanent dipole-dipole forces and hydrogen bonding, crucial for phenomena lik
0 views • 18 slides
Primary Bonding in Solids: Importance and Types
Exploring the fundamental concept of bonding in solids, this lecture delves into primary bonding, which includes covalent, ionic, and metallic bonds. The discussion highlights how different types of bonds impact the properties of materials such as metals, ceramics, and polymers. By understanding the
0 views • 19 slides
Ionic and Molecular Compounds in Chemistry
Discover the fundamental concepts of ionic and molecular compounds in chemistry with insights into the nature of elements, formation of compounds, and properties of ions. Explore the differences between ionic and covalent bonds, positive and negative ions, as well as examples of common everyday comp
0 views • 54 slides
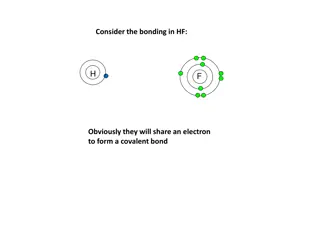
Bonding in HF Molecule
In HF bonding, hydrogen and fluorine share an electron to form a covalent bond. Fluorine, being more electronegative, attracts the bonding electrons more, resulting in a polar covalent bond. If hydrogen was less electronegative, the bonding electrons would shift further towards fluorine until an ion
0 views • 11 slides
Ionic Bonding and Octet Rule in Chemistry
Understanding the concept of ionic bonding and octet rule in chemistry is essential for grasping how atoms combine to form molecules through sharing or exchanging electrons. This process involves the formation of positive and negative ions held together by electrostatic attraction, leading to the cr
0 views • 12 slides
Ionic Bonding and Lattice Energy in Chemistry
Chemical bonds play a crucial role in holding atoms together in molecules. This course explores the concept of chemical bonding, focusing on ionic bonds and lattice energy. Topics covered include the different types of chemical bonds, such as electrovalent and coordinate bonds, as well as the models
0 views • 22 slides
Metallic Bonding and Its Properties
Metallic bonding involves the delocalization of electrons among metal atoms, creating a unique structure known as the electron sea. This structure allows for properties such as high melting points, conductivity of heat and electricity, malleability, and ductility. Metals are able to conduct heat and
0 views • 12 slides
Solid State Chemistry: Principles and Classification of Solids
Solid State Chemistry (CHEM 422) explores the principles and concepts governing the synthesis, structure, bonding, reactivity, and properties of solid state materials. The course delves into crystalline vs. amorphous solids, highlighting categories like ionic, molecular, metallic, and covalent solid
0 views • 24 slides
Ionic Bonding and Naming in Chemistry
Exploring the fundamentals of ionic bonding, naming conventions, and the Octet Rule in chemistry. Learn about Lewis structures, formation of ionic compounds, and the role of valence electrons in determining chemical properties. Discover how elements gain or lose electrons to achieve a full outer ene
0 views • 24 slides
Bonding in Ionic, Covalent, and Metallic Compounds
Explore the concepts of ionic, covalent, and metallic bonding through an investigation conducted by Vanderbilt Student Volunteers for Science. Learn about the different types of bonding, properties of ionic and molecular compounds, and the conductivity of metals. Discover the importance of determini
1 views • 15 slides
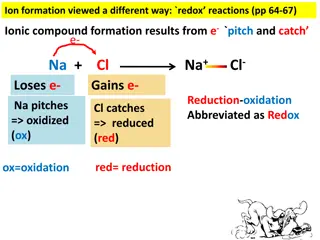
Redox Reactions and Ionic Compound Formation
Explore the concept of redox reactions through the process of ion formation, where elements lose or gain electrons to create ionic compounds. Learn about oxidation (ox) and reduction (red) in chemical reactions, and how to identify which elements lose or gain electrons based on charge changes. Disco
0 views • 14 slides