Solid State Chemistry: Principles and Classification of Solids
Solid State Chemistry (CHEM 422) explores the principles and concepts governing the synthesis, structure, bonding, reactivity, and properties of solid state materials. The course delves into crystalline vs. amorphous solids, highlighting categories like ionic, molecular, metallic, and covalent solids. Specifically, it breaks down the characteristics, properties, and structures of ionic and molecular solids, emphasizing factors like conductivity, melting points, solubility, and bonding types.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Solid state Chemistry (CHEM 422) Prof. Ayman Nafady King Saud University Chemistry Department
Week 1-2: Lectures Solid State Chemistry It is an in-depth examination of the principles and concepts underlying the synthesis, structure, bonding, reactivity, and physical properties of solid state materials. 2
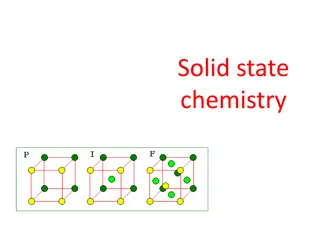
Solid State Solid is a physical state of matter which is characterized by strong intermolecular force of attraction and least intermolecular distance. The constituent particles are held together with strong forces which make this physical state compact and non-compressible. The constituent particles of solids cannot move from their position but can vibrate at their mean position. They are held together with strong intermolecular forces such as ionic or strong covalent bonding. Because of these strong forces, they have definite shapes and volumes. On the basis of ordered arrangement of particles, solids can be classified as crystalline and amorphous solid. 5
Crystalline solids Crystalline solids are classified according to their properties and structure into four Categories. These include: (1) Ionic solids (2) Molecular solids (3) Metallic solids (4) Covalent solids
(1)-Ionic Solids Ionic solids: are made of ions which sit in a 3D network of positive and negative ions. Are very hard but can be cleaved
Ionic solids properties Do not conduct electricity (no free ions or electrons) except when molten or aqueous (as ions are free to move) High melting point and boiling point because of very strong inter-particular attractions (electrostatic attractions) Soluble in polar substances Polar solvents overcome the electrostatic forces between ions
(2)-Molecular solids Molecular solids are made of molecules. There are strong bonds within the molecules (strong intra-molecular forces) but weak attractions between the molecules (weak inter molecular forces) Properties of Molecular solids: Have low melting and boiling points because of weak inter-molecular forces The molecules themselves are not broken up just moved apart from each other when molten
Molecular solids properties contd Do not conduct electricity - even when molten (no free ions or electrons) Insoluble in water and other polar substances Few are solid at room temperature eg. Dry ice
Polar and non- polar particles Molecular solids can be made from polar or non polar molecules. The inter- molecular forces between non-polar molecules are only weak van der Waals forces. Van der Waals attractions are the weak electrostatic attractions between atoms of different EN Polar molecules have dipoles and so have slightly stronger attractions between them meaning that the melting point of a polar molecular solid is a little higher because more energy is needed to overcome the slightly stronger forces.
(3)- Metallic solids Metals are made of positive nuclei held together by a sea of de- localised electrons (e- do not belong to any one nucleus) Forces between the particles are strong metallic bonds Malleable It can be hammered or bent into different shapes without breaking Ductile They can be drawn or pulled into a long strand of wire without breaking due to non-directional metallic bonds Atoms can slide over each other
Properties of metallic solids Good conductors of heat and electricity density and mobile electrons can transmit energy quickly High melting and boiling points due to strong metallic bonds Not soluble in any solvent The strength of metallic bonds varies consequently the melting and boiling different metals varies. due to and so points of
4- Network solids Made up of atoms connected by covalent bonds The intermolecular forces are covalent bonds as well. Characterized as being very hard with very high melting points and being poor conductors. 1. are infinitely long linear chains held together by van der Waals Properties: Low MP but longer chains have higher MP than shorter ones Do no conduct electricity Soft and flexible Eg. Polythene, rubber, plastics 1-D sloids (Linear chains)
2. 2-D Layers held together by weak van der Waals forces. Graphite is made of layers of covalently bonded C atoms. The layers are held together by weak van der Waals forces and have delocalised electrons between them. *this is very unusual and graphite is the only non- metal to conduct electricity
Properties of Graphite Conducts electricity because of delocalised electrons High melting point C atoms are held by strong covalent bonds Soft and greasy texture (used as a lubricant) as the layers slide past each-other easily weak van der Waals forces. Graphite has only 2-D hexagonal structure and therefore is not hard like diamond.
3. 3-D covalent network solids are made of atoms held together by strong covalent bonds. Diamond and silica (silicon dioxide) are two examples of covalent network solids. Diamond is the strongest substance known to man.
Amorphous Solids Properties No definite melting point - no crystal lattice to break Exhibit characteristic glass transition temperature, Tg Flow when subject to pressure over time Isotropic i.e. same properties in all direction 22