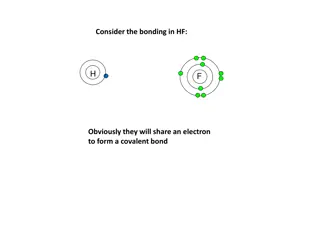
Chemical Bonding Concepts and Structures Explanation
Explore the concepts of chemical bonding through dot-and-cross diagrams for molecules like Antimony Chloride (SbCl3) and Boron Tribromide, along with explanations on ionic lattice structures, covalent bonds, and electrical conductivity in substances like Aluminium Fluoride (AlF3). Understand the shapes, structures, and bonding types in various molecules and compounds with detailed explanations.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
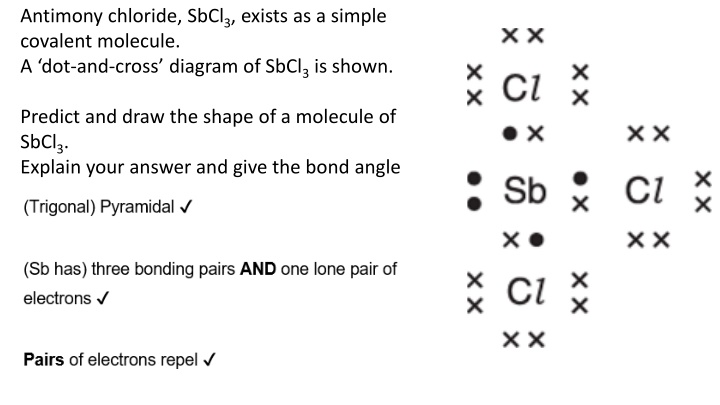
Antimony chloride, SbCl3, exists as a simple covalent molecule. A dot-and-cross diagram of SbCl3 is shown. Predict and draw the shape of a molecule of SbCl3. Explain your answer and give the bond angle
Draw a dot-and-cross diagram to show the bonding in CO2. Show outer electrons only.
Solid aluminium fluoride has a giant ionic lattice structure. Describe what is meant by the term ionic lattice, in terms of the type and arrangement of particles present. [2] Repeating pattern of oppositely charged ions Draw a dot-and-cross diagram for aluminium fluoride. Show outer electrons only. [2]
Solid boron tribromide has a simple molecular structure. The atoms are held together by covalent bonds. What is meant by the term covalent bond? A shared pair of electrons [1] Draw a dot-and-cross diagram to show the bonding in a boron tribromide molecule. Show outer electrons only. [1]
State whether the following substances conduct electricity when solid or molten, and explain your answers in terms of the particles involved: aluminium aluminium fluoride AlF3 conducts in molten states AlF3 because it has mobile ions Aluminium fluoride does not conduct when solid Solid aluminium fluoride has ions which are fixed (in position) OR ions are held (in position) OR ions are not mobile in an (ionic) lattice [5] Aluminium conducts in solid and molten states Aluminium has delocalised electrons boron tribromide Boron tribromide does not conduct in solid and molten states AND Boron tribromide has no mobile electrons OR no (mobile) ions OR no mobile charge carriers OR no mobile charged particles
Carbon monoxide contains a triple bond, and includes a dative covalent bond. Construct a dot-and-cross diagram to show the outer electron pairs in a molecule of carbon monoxide.
Solid barium chloride has a high melting point. Barium chloride dissolves in water to form a solution that can be used to test for sulfate ions. Draw a dot-and-cross diagram to show the bonding in solid barium chloride. Show outer electrons only. [2] A solution of barium chloride can be made in the laboratory using dilute hydrochloric acid. Suggest a compound that can be reacted with hydrochloric acid to make barium chloride. Barium hydroxide OR barium oxide OR barium carbonate [1]
Draw a dot-and-cross diagram to show the bonding in a nitrogen molecule. Show outer electrons only. [1]