Understanding Chemical Quantities: The Mole and Molar Mass
Explore the concept of chemical quantities through the mole and molar mass. Learn how to measure substances, calculate moles, find molar masses of compounds, and solve related problems in this informative chapter. Discover the significance of Avogadro's number, representative particles, and more in
9 views • 41 slides
Understanding Stoichiometry in Chemistry
Stoichiometry in chemistry involves calculating quantities of reactants and products in a chemical reaction based on a balanced equation. This process ensures the conservation of mass and atoms. Mole ratios are used as conversion factors, and steps such as converting to moles and applying the mole r
1 views • 25 slides
Stoichiometry Test Review Sheet
A comprehensive review sheet covering key concepts in stoichiometry, including molar ratios, coefficients in chemical equations, determining actual yield in reactions, identifying limiting reactants, calculating percentage yield, and understanding mole ratios in chemical reactions. The review provid
0 views • 11 slides
Understanding Mole Calculations in Chemistry
Explore various mole calculations in chemistry such as determining mass from moles, moles from mass, and comparing particles in different substances. Learn how to calculate the mass of substances, the number of particles, and perform calculations using balanced equations. Dive into concepts like mol
0 views • 49 slides
Understanding Gestational Trophoblastic Disease: Hydatidiform Mole & Choriocarcinoma
Gestational trophoblastic disease encompasses hydatidiform mole, invasive mole, and choriocarcinoma. This condition involves abnormal fertilization of the oocyte, resulting in various presentations including abnormal growth of the uterus, severe nausea, vaginal bleeding, and symptoms resembling hype
1 views • 9 slides
Exploring the Mole: A Fascinating Dive into Chemistry Concepts
Delve into the world of chemistry with a focus on the mole, stoichiometry, Avogadro's number, and the quantitative aspects of chemical reactions. Explore the significance of 6.02 x 10^23, learn about the origins of Avogadro's number, and grasp the vastness of a mole through intriguing analogies. Dis
0 views • 33 slides
Understanding the Mole and Avogadro's Law at Standard Temperature and Pressure (STP)
The lesson delves into the concepts of mole and Avogadro's law in relation to gases at standard temperature and pressure (STP). It explains how chemists track the number of gas particles using the mole unit, and highlights the significance of STP in gas calculations. Equal volumes of gases contain e
0 views • 9 slides
Reactor Sizing and Conversion in Chemical Engineering
This chapter explores the sizing of Continuous Stirred Tank Reactors (CSTR) and Plug Flow Reactors (PFR) using conversion values and overall conversion. It covers the definition of conversion, batch reactor design equations, design equations for flow reactors, and more. The content delves into the m
0 views • 17 slides
Colligative Properties in Solutions: Vapor Pressure, Freezing Point Depression, and Osmotic Pressure
Colligative properties such as vapor pressure lowering, freezing point depression, and osmotic pressure are characteristics of solutions that depend on the number of solute particles present. This text explores how these properties are related to the concentration of solute in a solution and how the
0 views • 14 slides
Fossorial Specimens: Mole and Caecilian Adaptations
Fossorial specimens, including the mole and caecilian, are uniquely adapted for subterranean burrowing. The mole, commonly known for its wedge-shaped head and reduced eyes, lives in tunnels and feeds on small worms and insects. On the other hand, the limbless caecilian, resembling a worm, thrives in
1 views • 25 slides
Update and Expansion of WMO X2004 CH4 Mole Fraction Scale
This study details the update and expansion of the WMO X2004 CH4 mole fraction scale, including reasons for changes, calibration of continental measurements, and the introduction of new primary standards calibrated with response curves. Various standards and measurements are discussed, and methods f
0 views • 19 slides
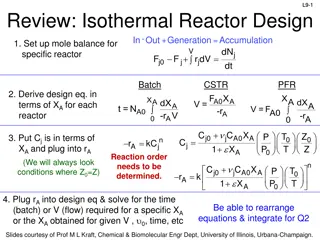
Chemical Reactor Design Review: Mole Balances, Rate Data Analysis, Method of Excess
This content provides a comprehensive review of isothermal reactor design, including setting up mole balances, deriving design equations in terms of conversion, analyzing rate data to determine reaction order and rate constant, and applying the method of excess to evaluate reaction kinetics. Detaile
0 views • 21 slides
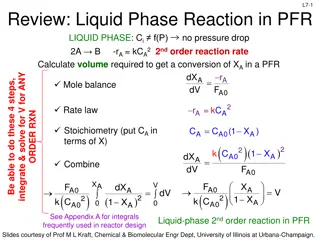
Reactor Design Principles for Liquid and Gas-Phase Reactions
This material covers the design principles for liquid and gas-phase reactions in continuous flow reactors such as PFR (Plug Flow Reactor) and PBR (Packed Bed Reactor). It includes calculations for volume required to achieve a specific conversion, catalyst weight needed, and considerations for ideal
0 views • 21 slides
The Equivalence of Egg Math and Mole Math
Explore the interesting comparison between egg math and mole math, where 1 egg is equivalent to one practical counting unit in the world of chemistry. Discover how a chemist's dozen relates to the Avogadro's number and molecular weight, highlighting the parallel between egg quantities and chemical e
1 views • 21 slides
Lois Ehlert - A Creative Journey Through Art and Storytelling
Lois Ehlert, born on November 9th, 1934, in Beaver Dam, Wisconsin, is a renowned artist and author currently residing in Milwaukee. Her unique upbringing surrounded by creativity led her to study English, Psychology, and Graphic Design. Ehlert's career transitioned from creating illustrations for ot
0 views • 14 slides
Assay of Aspirin by Indirect Acid-Base Titration
Indirect titration, also known as residual titration, is an analytical technique used to determine the weight of an unknown sample by employing excess standard solution. In the case of aspirin, which is a weak acid undergoing slow hydrolysis, back titration with NaOH and HCl is utilized to overcome
1 views • 13 slides
Understanding Solution Composition in Chemistry
Exploring the properties of solutions, this content delves into measuring solution composition using techniques like molarity, mass percent, and mole fraction. Through examples, it explains how to calculate the weight percent of solutes in solutions, offering a comprehensive guide on understanding e
0 views • 66 slides
Introduction to Chemical Reaction Engineering (CRE)
Chemical Reaction Engineering (CRE) focuses on studying the rates and mechanisms of chemical reactions, as well as designing reactors for these reactions. The field involves understanding balances in terms of molar flow rates, mole balances, rate laws, stoichiometry, and membrane reactors. Membrane
0 views • 20 slides
Chapter 2. Atoms, Molecules, and Ions
Explore the historical timeline of atomic theory advancements, from Demokritos' initial concept of "atomos" to Dalton's Atomic Theory, Thomson's discovery of the electron, and modern breakthroughs by scientists like Rutherford and Lawrence. Avogadro's contributions to the mole concept and gas laws a
0 views • 46 slides
Understanding Factors Affecting Enzyme Activity in Biochemistry
Enzyme assays measure substrate conversion to product under varying conditions like cofactors, pH, and temperature. Enzyme velocity represents the rate of a catalyzed reaction, typically reported as V0. Enzyme activity is expressed as mol of substrate transformed per minute, with enzyme unit and kat
0 views • 18 slides
Evolution of the International System of Units (SI) and Its Base Units
The International System of Units (SI) was introduced in France in 1840 and became globally accepted by the mid-20th century. SI is mandatory in Estonia since 1982, with base units including meter, kilogram, second, ampere, kelvin, candela, and mole. Each base unit is defined by specific physical co
0 views • 22 slides
Understanding the Mole Concept in Chemistry
Delve into the world of chemistry with the Mole Concept, exploring molar mass, Avogadro's number, representative particles, and more. Learn how to determine molar mass for compounds and grasp the significance of a mole in chemical calculations.
0 views • 27 slides
Understanding Unit Conversion and Mole Concept in Chemistry
Explore the concepts of unit conversion and the mole in chemistry, including how to convert between different units, relate mass to atoms and molecules, calculate molar mass, and perform conversions involving substances like chalk and sodium hydroxide. Discover the importance of dimensional analysis
0 views • 12 slides
Understanding Mole Calculations and Its Applications
Dive into the world of mole calculations with Chapter 5 homework assignments, exploring concepts such as mole weight, the dozen concept, and sweet mole calculations. Discover the significance of moles in chemistry and how they relate to everyday scenarios like calculating the weight of eggs and unde
1 views • 28 slides
Understanding Thermal Physics and Gas Laws in Physics Class
Explore the concepts of thermal physics and gas laws in today's physics class. Learn about the transfer of heat, the mole concept, atomic and molar masses, pressure, standard pressure and temperature (STP), and ideal gases. Delve into calculations involving sample materials like copper and water to
0 views • 20 slides
Understanding the Kinetic Theory of Gases and Ideal Gas Model
Explore the Kinetic Theory of Gases, the Molecular Model of Ideal Gases, and concepts like pressure, temperature, and heat capacity. Understand the fundamental properties of gases, including the distribution of molecular speeds and equations that describe gas behavior. Learn about the mole concept,
0 views • 23 slides
Understanding Metric System and Sig Figs in Chemistry
Explore the fundamentals of the metric system, significant figures, and unit conversions in the context of chemistry. Gain insights into the mole concept, prefixes, and methods for handling calculations with appropriate precision. Get ready to tackle questions and operations related to sig figs, mea
0 views • 17 slides
Acid-Base Titration Lab Procedure & Calculations
Conduct an acid-base titration experiment to determine the molarity of an unknown NaOH solution using HCl as the titrant. Follow the outlined steps, record data, perform calculations, and analyze the results to understand the principles of this chemical reaction. Learn about indicators, mole ratios,
0 views • 7 slides
Upgrade Plans for Run-2C Project Activities
The upgrade plans for the Run-2C project involve a series of detailed tasks including inserting a snorkel tube, installing micron disks in the LAr filter, attaching filters and mole sieve, regenerating filters, and testing various components. Additionally, there are plans for replacing sintered meta
0 views • 13 slides
Understanding Stoichiometry and The Mole Concept in Chemistry
Explore the fundamentals of stoichiometry and the mole concept in chemistry, including conversions between moles and particles, molar mass calculations, and gram mole conversions. Learn how to determine the number and kinds of atoms in chemical formulas and understand the significance of Avogadro's
0 views • 12 slides
Understanding Toxicity: Mole-Mass Conversions in Chemistry
Explore the toxic nature of arsenic compounds and learn how to convert between moles and mass in this chemistry lesson. Understand the relationships between mass, moles, and the number of particles in a substance, and practice converting moles to mass and vice versa. Work in pairs to delve into the
0 views • 10 slides
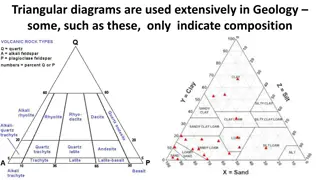
Compositional Analysis of Rock-Forming Minerals Using Triangular Diagrams
Geologists frequently utilize triangular diagrams to determine mineral compositions in the Al2O3-SiO2-K2O system. This analysis helps identify stable minerals under Earth-surface conditions, considering primary weathering minerals. The process involves calculating mole percentages of oxides in miner
1 views • 25 slides
Understanding the Concept of Molar Mass in Chemistry
Explore the concept of molar mass in chemistry, including the definition of the mole, Avogadro's number, calculations for molar mass of elements and compounds, and examples of determining molar mass. Discover how to find the molar mass of various compounds through practical examples.
0 views • 39 slides
Understanding the Mole Concept in Chemistry
Explore the fundamentals of the mole concept in chemistry, including calculations involving molecular formulas, reagent masses, percentage yields, gas volumes, concentrations, and balanced chemical equations. Learn about the definition of a mole, calculating the number of moles, and converting mass
0 views • 23 slides
Understanding Moles in Chemistry
Matter is composed of various particles, and chemists use the concept of moles as a unit of measure to quantify the number of particles in a substance. One mole is equal to 6.02 x 10^23 representative particles of a substance, known as Avogadro's number. Moles are versatile and applicable to differe
0 views • 25 slides
Understanding Standard Molar Enthalpies of Formation
Formation reactions involve substances being created from elements in their standard states, with the enthalpy change known as the standard molar enthalpy of formation (Hf). This enthalpy represents the energy released or absorbed when one mole of a compound is formed from its elements in their stan
0 views • 13 slides
Understanding Molar Mass and Conversions in Chemistry
Explore the concept of molar mass, moles of atoms, and conversions between mass and moles in chemistry through an engaging lesson on toxins, stoichiometry, solution chemistry, and acids and bases. Learn how to calculate molar mass, describe the magnitude of a mole of a substance, and conduct simple
0 views • 10 slides
Understanding Chemical Quantities: The Mole Concept and Molar Mass
Chemists use the mole concept to relate mass and the number of atoms in chemical reactions. Avogadro's number, molar mass, stoichiometry, and energy changes in reactions are key concepts explored in this chapter. The mole is a vital unit in chemistry, enabling scientists to quantify substances and m
0 views • 77 slides
Understanding Chemical Quantities: The Mole Concept in Chemistry
Exploring the concept of the mole in chemistry, this chapter delves into measuring matter in terms of count, mass, or volume. Avogadro's number is defined, and representative particles such as atoms, molecules, and ions are discussed. The calculation of molar mass for elements and compounds is expla
0 views • 41 slides
Best Thread vein removal in Westfield Sole
Are you looking for the Best Thread vein removal in Westfield Sole? Then contact Aesthetics By Emma. With a personalized approach to skincare and beauty, Emma offers a range of services, including anti-wrinkle injections, lip fillers, mole removal, t
0 views • 6 slides