Chapter 2. Atoms, Molecules, and Ions
Explore the historical timeline of atomic theory advancements, from Demokritos' initial concept of "atomos" to Dalton's Atomic Theory, Thomson's discovery of the electron, and modern breakthroughs by scientists like Rutherford and Lawrence. Avogadro's contributions to the mole concept and gas laws are also highlighted, showcasing the progression of understanding atoms, molecules, and ions in chemistry.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Chapter 2 Atoms, Molecules, and Ions DE Chemistry Dr. Walker
Timeline B.C. 400 B.C. Demokritos and Leucippos use the term "atomos 2000 years of Alchemy (other elements to gold) 1500's Georg Bauer: systematic metallurgy Paracelsus: medicinal application of minerals 1600's Robert Boyle: The Skeptical Chemist. Quantitative experimentation, identification of elements 1700s' Georg Stahl: Phlogiston Theory Joseph Priestly: Discovery of oxygen Antoine Lavoisier: The role of oxygen in combustion, law of conservation of mass, first modern chemistry textbook
Timeline 1800's Joseph Proust: The law of definite proportion (composition) John Dalton: The Atomic Theory, The law of multiple proportions Joseph Gay-Lussac: Combining volumes of gases, existence of diatomic molecules Amadeo Avogadro: Molar volumes of gases Jons Jakob Berzelius: Relative atomic masses, modern symbols for the elements Dmitri Mendeleyev: The periodic table J.J. Thomson: discovery of the electron Henri Becquerel: Discovery of radioactivity 1900's Robert Millikan: Charge and mass of the electron Ernest Rutherford: Existence of the nucleus, and its relative size Meitner & Fermi: Sustained nuclear fission Ernest Lawrence: The cyclotron and trans-uranium elements
Daltons Atomic Theory (1808) The Short Version Each element is made up of tiny particles called atoms The atoms of a given element are identical Chemical compounds are formed when atoms combine with each other. A given compound always has the same relative numbers and types of atoms Chemical reactions involve reorganizations of the atoms. The atoms themselves are not changed in a chemical reaction
Avogadro (Yes, him again) At the same conditions of temperature and pressure, equal volumes of different gases contain the same number of particles. Relates to the mole concept 1 mole = 22.4 L (@ STP) = molar mass (grams) = 6.02 x 1023 particles Relates to gas laws 1 mole not equal to 22.4 L at other conditions Find volume by using ideal gas law
Thomson (1897) Cathode Ray Tube Experiment Determined the charge to mass ratio of the electron Reasoned that all atoms must contain electrons Reasoned that all atoms must contain positive charges
Thomsons Atomic Model Thomson believed that the electrons were like plums embedded in a positively charged pudding, thus it was called the plum pudding model. J.J. Thomson
Millikan Mass of Electrons 1909 Robert Millikan determines the mass of the electron using charged oil droplets Mass of the electron is 9.109 x 10-31 kg
Fundamental Chemical Laws Law of Conservation of Mass "Mass is neither created nor destroyed" Translation: In ordinary chemical reactions, the total mass of the reactants is equal to the total mass of the products Why equations need to be balanced Basis for stoichiometry, percent yield, etc.
Fundamental Chemical Laws Law of Definite Proportion "A given compound always contains the same proportions of elements by mass" Translation: Compounds have an unchanging chemical formula Different Formula, different compound
Fundamental Chemical Laws Law of Multiple Proportions (Dalton) When two elements form a series of compounds, the ratios of the masses of the second element that combine with one gram of the first element can always be reduced to small whole numbers Translation: Sometimes two elements can come together in more than one way, forming compounds with similar, though not identical formulas Example: Hydrogen and Oxygen 2 H2 + O2 2 H2O H2 + O2 H2O2
Radioactivity Gamma ( ) rays - high energy light Beta ( ) particles - high speed electrons Alpha ( ) particles helium nucleus, particle with a 2+ charge
Rutherford Gold Foil Experiment Alpha particles are helium nuclei Particles were fired at a thin sheet of gold foil Particle hits on the detecting screen (film) are recorded
Gold Foil Experiment Some particles were greatly deflected ("like a howitzer shell bouncing off of a piece of paper") Could not have been deflected by electrons or single protons Must have been deflected by a positively charged object of substantial mass Supported concept of a small, central, positive nucleus where most of the atom's mass was concentrated Disproved Thomson's "plum pudding" model
Atomic Scale Nucleus is roughly 1/105 the diameter of the atom Most of the mass of the atom is in the nucleus (protons and neutrons) Electrons are found outside of the nucleus (the electron cloud) Most of the volume of the atom is empty space Nucleus = 10-15 m Atom = 10-10 m
Quarks The True Fundamental Particle Protons and neutrons are NOT fundamental particles. Protons are made of two up quarks and one down quark. Neutrons are made of one up quark and two down quarks. Quarks are held together by gluons
Atomic Particles Subatomic Particle Mass Location Charge Proton 1.67 x 10-27 kg Nucleus + 1 Neutron 1.67 x 10-27 kg Nucleus 0 Electron 9.1 x 10-31 kg (much smaller) Orbitals (outside nucleus) - 1
Atomic Number Atomic Number = # protons = # electrons (when neutral) Mass Number = protons + neutrons Nuclide p+ n0 e- Mass # Oxygen - 10 - 33 42 - 31 15
Atomic Number Atomic Number = # protons = # electrons (when neutral) Mass Number = protons + neutrons Nuclide p+ n0 e- Mass # Oxygen - 18 8 10 8 18 Arsenic - 75 33 42 33 75 Phosphorous - 31 15 16 15 31
Atomic Masses Atomic mass is the weighted average of all the naturally isotopes of that element. Isotope Symbol Composition of % in nature the nucleus 6 protons 6 neutrons Carbon-12 12C 98.89% Carbon-13 13C 6 protons 7 neutrons 1.11% Carbon-14 14C 6 protons 8 neutrons <0.01% Carbon = 12.011
Isotopes Isotopes Same element Same # protons, varying # neutrons Isotope Protons Electrons Neutrons Nucleus Hydrogen 1 (protium) 1 1 0 Hydrogen-2 (deuterium) 1 1 1 Hydrogen-3 (tritium) 1 1 2
From Atoms to Compounds DE Version
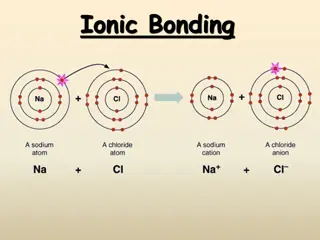
Molecules and Ions Chemical Bonding Covalent Bonding Sharing of electrons Ionic Bonding Transfer of electrons between elements which form ions
Covalently Bound Molecules Compounds with covalent bonds referred to as molecules Formulas represent each atom present in the molecule Structural formula Space filling model Ball and Stick model
Ions Cation: A positive ion formed by losing electrons Mg2+, NH4+ Anion: A negative ion formed by gaining electrons Cl , SO42 Remember, atoms want a full valence shell ! Ionic Bonding: Force of attraction between oppositely charged ions. Ionic compounds form crystals, so their formulas are written empirically (lowest whole number ratio of ions).
Valence Electrons and Charge Group 1 2 13 14 15 16 17 18 Valence Electrons 1 2 3 4 5 6 7 8 Lose or Gain Lose 1 Lose 2 Lose 3 Either/ or Gain 3 Gain 2 Gain 1 Neither Charge +1 +2 +3 +4 or - 4 -3 -2 -1 0 Keep in mind: -This is a generalization. There are numerous exceptions. - Remember, many (not all) transition metals can have multiple charges.
Common Ions This is not a complete list, but includes general examples
Binary Ionic Compounds Binary = 2 elements Cation first, use name of the element Anion second, use root of the element name and add ide Na+ and Cl- makes NaCl = sodium chloride Ca2+ and Br- makes CaBr2 = calcium bromide K+ and O2- makes K2O = potassium oxide As before, you will be expected to go in both directions Name to formula AND formula to name!
Rules of Thumb Naming Polyatomics Find the "ate" ion (sulfate, for instance) sulfate = SO42- The "ite" ion always has one less oxygen than the "ate" ion sulfite = SO32- The prefix "per" (think hyper, meaning "above") is used with the "ate" prefix to indicate one more oxygen than the "ate" ion persulfate = SO52- The prefix "hypo" (meaning "under" or "below") is used with the "ite" prefix to indicate one less hyposulfite = SO22-
Rules of Thumb Naming Polyatomics Examples Chlorate and Nitrate have 3 oxygens Per - ate has 4 oxygens (1 more) ite has 2 oxygens (1 less) hypo ite has 1 oxygen (1 less than ite )
Practice - Answers a. KI b. CaO C. GaBr3
Binary Ionic Compounds Transition Metals* Same rules apply with one exception Use roman numeral to show positive charge on cation Fe3+ and Cl- makes FeCl3 = iron (III) chloride Cu+ and F- makes CuF = copper (II) fluoride Pb4+ and O2- makes PbO2 = lead (IV) oxide* Remember, reduce numbers in formulas when possible *Metals such as tin and lead are not transition metals, yet they can have multiple charges
Ionic Compounds - Polyatomics Cation first List cation, whether it is an element or polyatomic Anion second (as usual) Use ide for elements, list name if polyatomic Use parentheses for multiple polyatomics, as shown below NH4+ and S2- makes (NH4)2S, ammonium sulfide Ca2+ and C2H3O2- makes Ca(C2H3O2)2, calcium acetate
Naming Acids Binary Acids (two elements - hydrogen + one other) prefix "Hydro" + root of second element + "ic" suffix HCl = hydrochloric acid HBr = hydrobromic acid
Naming Acids Oxyacids acid including oxygen (from polyatomic) If the acid contains an anion whose name ends in "ate": Use root of anion name and an "ic" ending H2SO4 = sulfuric acid (SO4 = sulfate) H2CO3 = carbonic acid (CO3 = carbonate) If the acid contains an anion whose name ends in "ite": Use the root of the anion name and an "ous" ending H2SO3 = sulfurous acid (SO3 = sulfite) HClO = hypochlorous acid (ClO = hypochlorite)
Covalent Bonding Naming covalent compounds: Use prefixes
Molecular Compounds Compounds between two nonmetals First element in the formula is named first. Keeps its element name Gets a prefix if there is a subscript on it Second element is named second Use the root of the element name plus the -ide suffix Always use a prefix on the second element SO2 Sulfur dioxide N2O3 Dinitrogen trioxide SiBr4 Silicon tetrabromide
Answers a. SF2 B. SF6 C. NaH2PO4 D. Li3N E. Cr2(CO3)3 F. SnF2 G. NH4C2H3O2 H. NH4HSO4 i. Co(NO3)3 J. HgCl K. KClO3 L. NaH
Answers a. Cesium fluoride c. Silver sulfide* e. Titanium (IV) oxide b. Lithium Nitride d. Manganese (IV) oxide f. Strontium phosphide * Despite being a transition metal, silver only forms one cation a. Barium sulfate c. Potassium permanganate b. Sodium nitrite d. Potassium dichromate
Problem Set Chapter 2 # 7, 11, 12, 22, 24, 42, 44, 46, 50, 54, 56, 60, 64, 66