Geometrical Isomerism in Octahedral Complexes: A Comprehensive Overview
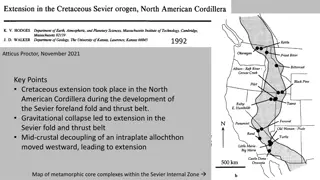
Geometrical isomerism in octahedral complexes is a fascinating phenomenon arising from different geometric arrangements of ligands. This type of isomerism is prevalent in coordination numbers 4 and 6, leading to two main types of geometric isomers. Examples of cis-trans and mer-fac isomers in MA2B4 and MA3B3 complexes are illustrated, showcasing the unique arrangements of ligands in octahedral geometry.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
GEOMETRICAL ISOMERISM IN OCTAHEDRAL COMPLEXES Mary Sarah Cherian Associate Professor Department of Chemistry Mar Thoma College Tiruvalla
GEOMETRICAL ISOMERISM Geometrical isomerism is a type of isomerism arises in heteroleptic complexes due to different possible geometric arrangements of the ligands. Important examples of this behavior are found with co-ordination numbers 4 and 6. There are two simples types of geometric isomerism possible for octahedral complexes.
The first type of geometrical isomerism exists for complexes of the type MA2B4. Two geometrical isomers are also possible for complexes of the type MA2B4.
GEOMETRICAL ISOMERS OF MA2B4 TYPE a) The A ligands in MA2B4 may be either next to each other called cis isomer. b) The A ligands in MA2B4 areon opposite apexes of the octahedron called trans isomer. Complexes of this type were studied by Werner,who showed that the praseo and violeo complexes of tetraaminedichlorocobalt(III) were of this type .
Examples of cis and trans octahedral isomers of tetraaminedichloro cobalt(III)
The second type of geometrical isomerism exists for the complexes of the type MA3B3. Two geometric isomers are also possible for MA3B3 type.
GEOMETRICAL ISOMERS OF MA3B3 TYPE a) The ligands of one type may form an equilateral triangle on one of the faces called facial isomer or fac isomer. b) The ligands may span three positions such that two are opposite to each other called meridional isomer or mer isomer.
Mer-fac isomers are easier to notice than cis- trans isomers in the sense that they only exist in octahedral geometry. Just like cis-trans isomers,mer-fac isomers are determined based on whether or not the ligands exist on the other side.
Examples of facial and meridional octahedral isomers of triaminetrinitro cobalt(III).