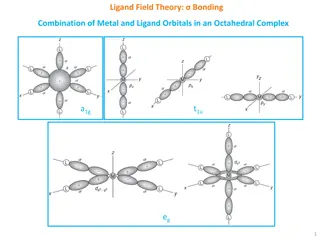
Understanding Enemark-Feltham Notation in Iron-Nitrosyl Complexes
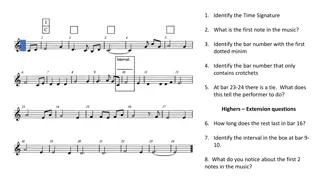
Iron-Nitrosyl complexes are redox non-innocent, with NO exhibiting multiple redox states. Enemark-Feltham Notation helps in determining metal-ligand interactions and oxidation states. Detailed information on NO ligands, bonding characteristics, and methods for analyzing iron-NO systems are discussed.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
5-ish Slides about Enemark- Feltham Notation Kyle Grice DePaul University November 2018
Iron Nitrosyl Complexes NO ligands are redox non-innocent, which means NO has multiple accessible redox states under common conditions In addition, Iron has common several redox and spin-states (Fe2+vs. Fe3+, high spin or low spin).
NO (Yes?) As a neutral molecule, NO is a radical with one unpaired electron Bond Order: 2.5 Bond Length: 115 pm NO has been found to be a neurotransmitter and vasodialator There has been significant research in NO-releasing molecules (NORMs) for medical applications.
Basics of Enemark-Feltham {M-L}X or {Fe-NO}X where X is the sum of the metal valence and ligand * electrons. NO+ has no * electrons, NO has 1, and NO- has 2. Iron can be high spin or low spin in several oxidation states! Nitroprusside is a blood-pressure lowering compound: Enemark-Feltham Notation: {Fe-NO}6
How do we get more detailed info? Often it is hard to determine the exact oxidation state. information can sometimes be obtained from the NO ligand: NO+ ligands have shorter bonds because they have formally triple N-O bonds. They tend to be linear. NO- are bent due to the extra lone pair on the nitrogen. NO is in-between these two extremes in term s of length and angle IR frequencies also help: Linear: 1650 1900 cm-1 Bent: 1520 1690 cm-1 Linear Fe-N-O bonding and a short N-O distance. The molecule is diamagnetic too! This is low spin Fe2+ with a NO+ ligand.
Beyond Enemark-Feltham To deconvolute the oxidation state of the iron and the NO, various methods can be used: Evidence from X-ray structures and IR DFT calculations Fe K-edge XAS Fe Mossbauer