Transition Metals Organometallic Compounds Overview
Transition metals bonded with organometallic compounds like metal alkyls, aryls, and hydrides are common in chemistry. Ligands are crucial for stabilizing these complexes, with carbon-based ligands exhibiting diverse binding modes based on the metal's hybridization state. Preparation methods for tra
1 views • 25 slides
Crystal Field Theory in Transition Metal Complexes
Crystal Field Theory (CFT) explains the colors and magnetic properties of transition metal complexes. It focuses on the energy changes in d-orbitals of metal ions caused by surrounding ligands. This theory, developed in 1929, provides insights into the bonding interactions in complex compounds. The
10 views • 44 slides
Pharmaceutical Chemistry Analgesic Agents Narcotic Analgesics
Analgesic agents play a crucial role in managing pain, ranging from mild to severe, with different categories such as opioids, NSAIDs, and triptans. The origin of pain can vary from physiological to neuropathic causes. Opioids target opioid receptors, with discoveries in endogenous ligands like enke
4 views • 49 slides
Ligands and Metal Carbonyl Complexes: A Comprehensive Overview
Explore the classification of ligands in metal complexes, including alkyl, aryl, and carbonyl ligands. Learn about the unique properties of carbonyl ligands, their preparation methods, and the molecular orbital diagram illustrating CO-metal bonding interactions.
5 views • 35 slides
Organometallic Chemistry III: Transition Metal Complexes and Homogeneous Catalysis
Explore the reactivity of transition metal complexes, including bond metatheses and various reactions. Learn about orbital considerations, synthesis, and spectroscopic properties of organometallic complexes. The course covers basics from AC1, focusing on ligands, electron counting, and MO diagrams.
6 views • 8 slides
Narcotic Analgesics and Opiates: History, Mechanisms, and Uses
Delve into the world of narcotic analgesics and opiates, exploring the history of opium poppy, morphine derivatives, opioid compounds, and the pharmacology mechanisms of action. Discover the uses of opiates in analgesia, preanesthetic medication, and more, alongside the endogenous ligands involved.
8 views • 55 slides
Crystal Field Theory in Chemistry
Crystal Field Theory (CFT) explains how electron orbital degeneracies, particularly d or f orbitals, are affected by a static electric field generated by neighboring anions. In CFT, the metal ion is considered positive while ligands are negative charges, leading to attractive and repulsive forces af
0 views • 13 slides
Hard and Soft Acids and Bases (HSAB Principles) by Dr. Gurpreet Kaur
Delve into the world of Hard and Soft Acids and Bases (HSAB) with Dr. Gurpreet Kaur as she explains the characteristics of hard and soft acids, Pearson's HSAB principle, applications such as predictions of coordination in complexes, poisonings of metal catalysts, and the classification of acids and
2 views • 17 slides
Coordination Compounds and Ligands in Chemistry
Coordination compounds involve ligands that donate electron pairs to central metal ions. Ligands can be categorized based on the number of donor atoms they contain, such as mono-, bi-, tri-, tetra-, penta-, and hexadentate ligands. Each type of ligand has the ability to form bonds with the central m
3 views • 15 slides
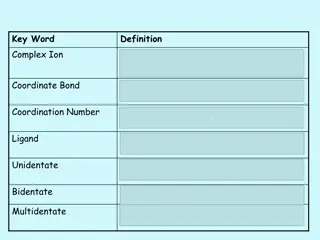
Complex Ions and Coordinate Bonds in Chemistry
Complex ions in chemistry are formed when transition metals or their ions bond with ligands through coordinate bonds. Ligands utilize their lone pairs of electrons to form dative covalent bonds with transition metals, determining the coordination number of the cation. Complex ions play a crucial rol
1 views • 29 slides
Enemark-Feltham Notation in Iron-Nitrosyl Complexes
Iron-Nitrosyl complexes are redox non-innocent, with NO exhibiting multiple redox states. Enemark-Feltham Notation helps in determining metal-ligand interactions and oxidation states. Detailed information on NO ligands, bonding characteristics, and methods for analyzing iron-NO systems are discussed
0 views • 6 slides
Geometrical Isomerism in Octahedral Complexes: A Comprehensive Overview
Geometrical isomerism in octahedral complexes is a fascinating phenomenon arising from different geometric arrangements of ligands. This type of isomerism is prevalent in coordination numbers 4 and 6, leading to two main types of geometric isomers. Examples of cis-trans and mer-fac isomers in MA2B4
5 views • 10 slides
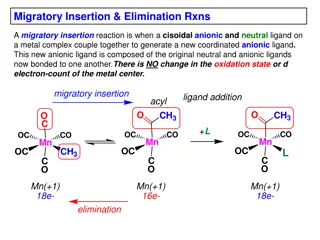
Insights into Migratory Insertion and Elimination Reactions in Metal Complexes
Migratory insertion in metal complexes involves the coupling of a cisoidal anionic and neutral ligand to form a new coordinated anionic ligand without changing the metal center's oxidation state or d-electron count. This process requires cisoidality between the reacting ligands and a vacant coordina
0 views • 19 slides
Role of Adhesion Molecules in Immune Response to SARS-CoV-2 Engagement
Engagement of SARS-CoV-2 triggers the complement system and TLR 7 in neutrophils and macrophages, leading to the release of inflammatory cytokines and chemotactic factors. This results in the upregulation of adhesion molecules on blood capillary endothelial cells and leukocytes, facilitating leukocy
3 views • 12 slides
Affinity Chromatography: A Breakthrough in Biochemical Research
Affinity chromatography, developed in the 1930s by A. Wilhelm Tiselius, is a vital technique for studying enzymes and proteins. It relies on the specific affinity between biochemical compounds and utilizes matrices like agarose for binding sites. Ligands such as amino and hydroxyl groups play crucia
1 views • 27 slides
Bioinorganic Chemistry: Connecting Inorganic and Biochemistry
Bioinorganic Chemistry bridges the gap between inorganic chemistry and biochemistry, understanding the vital role of inorganic elements in living systems. This interdisciplinary field delves into the structure, function, and exploitation of metal ions in biological processes, emphasizing their inter
0 views • 47 slides
Coordination Numbers in Inorganic Compounds: Geometries and Structures
In inorganic coordination complexes, the coordination number refers to the number of atoms bonded to the central atom. Common geometries include octahedral, tetrahedral, and square planar, depending on the type and number of ligands. Transition metal complexes exhibit different coordination numbers
2 views • 8 slides
Coordination Chemistry: Structures, Isomers, and Naming
Exploring coordination chemistry involves understanding structures, isomers, naming conventions, and common coordination numbers, all essential in studying coordination compounds. Coordination compounds consist of central metals, ligands, and charge balancing ions. Naming involves listing cations, l
1 views • 46 slides
Crystal Field Theory and Color Exhibited by Coordination Compounds
Crystal Field Theory (CFT) explains the colors exhibited by coordination compounds based on the absorption of light and electron transitions in d-orbitals. The theory describes how ligands interact with transition metal ions, causing the d-orbitals to split in energy levels. This split results in th
0 views • 30 slides
Insights into Coordination Chemistry Elements and Complexes
Transition elements with d or f electrons possess unique properties, play crucial roles in biological processes, and form colorful complexes with ligands. Occurring widely in nature, these elements have varied oxidation states and coordination numbers. Werner's formulation sheds light on primary and
0 views • 41 slides
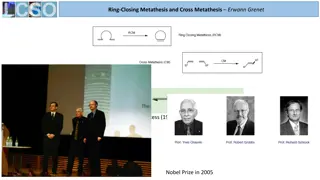
Advances in Ring-Closing Metathesis and Cross-Metathesis Catalysts
Recent developments in metathesis catalysts, focusing on Molybdenum and Ruthenium-based catalysts. Comparison of Schrock and Grubbs catalysts, ligands, and new modified catalysts. Details on activity, stability, and group tolerance. The potential of new catalysts like Piers II, Grubbs III, nitro-Gre
0 views • 6 slides
Cell Communication: Signaling Mechanisms and Types
Cell communication plays a crucial role in biological processes, involving intercellular and intracellular signaling through ligands and receptors. This communication occurs via various forms of chemical signaling, including paracrine, endocrine, autocrine, and direct signaling methods. Each type of
0 views • 27 slides
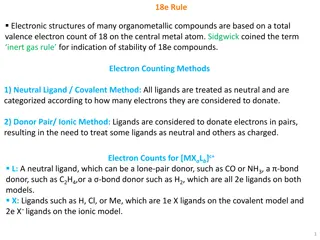
The 18e Rule in Organometallic Compounds
The 18e rule dictates the electronic structures of many organometallic compounds, emphasizing a total valence electron count of 18 on the central metal atom for stability. Electron counting methods like the Covalent and Ionic models assist in determining the electron distribution among ligands. The
0 views • 8 slides
Coordination Complexes and Transition Metals
Today's lecture covers transition elements, coordination complexes, ligand types, geometries, naming, isomers, and bonding in coordination complexes. Transition metals form coordination complexes with metal ions, ligands, and counter ions. The types of ligands include monodentate and bidentate ligan
1 views • 24 slides
Chelation Chemistry: Structural Requirements, Ligands, and Applications
Chelation chemistry involves the formation of specific complexes known as chelates, characterized by ligands that coordinate with a central metal ion. This article explores the structural requirements for chelate formation, the role of chelating agents like EDTA and DMG, and the difference between m
0 views • 27 slides
Nuclear Receptor-Mediated Toxicity: Molecular Insights and Implications
TOXICOLOGY research on Nuclear Receptor-Mediated Toxicity by Prof. Zdeněk Dvořák delves into the evidence, molecular properties, structures, and signaling pathways of nuclear receptors such as Aryl Hydrocarbon Receptor (AhR). Detailed information on the impact of AhR-mediated toxicity, including
0 views • 19 slides
Ligands for Nanoparticle Surface Effects
Design and Synthesis of Ligands for studying Nanoparticle Surface Charge and Distribution, including examples of monomeric and polymeric ligands. Critical design elements of the ligand library are discussed, emphasizing scalable synthesis and key features. Initial synthesis challenges are noted, wit
0 views • 14 slides
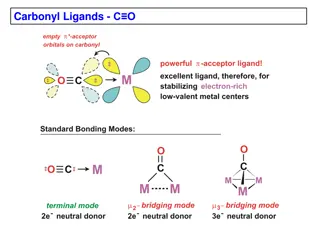
Carbonyl Ligands -
Metal-carbonyl complexes are important in coordination chemistry. This content covers examples of neutral binary metal carbonyls, MO diagrams, CO-metal bonding interactions, IR stretching frequencies, and electronic effects on CO. The text explores the nature of metal-CO bonding, the positioning of
0 views • 13 slides
Lead Compound Development in Pharmaceutical Chemistry
In organic pharmaceutical chemistry, discovering lead compounds is crucial for drug development. Natural ligands, combinatorial synthesis, computer-aided design, and fragment-based lead discovery are key techniques used to design novel compounds that interact with specific targets effectively.
0 views • 23 slides
Classification of Opioids and Their Mechanisms
In-depth analysis of opioid classification (natural, semi-synthetic, synthetic), receptors, endogenous ligands, mechanisms of action, mood alterations, CNS effects, and their impact on MAC. Images included.
0 views • 20 slides