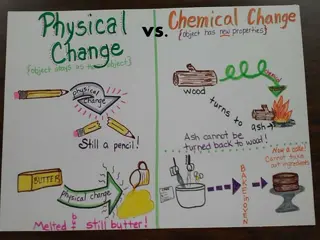Introduction to Physical and Chemical Changes in Chemistry
Explore the concepts of physical and chemical changes in chemistry, including examples and distinctions between properties. Discover the SI units of mass, density, and temperature conversions, as well as significant figures and scientific notation.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
Chapter 1: The Study of Change Chapter 1: The Study of Change Dr. Nouf Alotaibi
Which of the following would NOT be a physical change? a. freezing water to make ice cubes. b. melting gold to make jewelry. c. burning a piece of paper. d. tearing a piece of aluminum foil. Which of the following would be a chemical change? a. perfume evaporating on your skin. b. melting butter for popcorn. c. burning sugar. d. a hot glass breaking when placed in cold water.
Which of the following is an example of a physical property? A) Combustibility B) Corrosiveness C) Explosiveness D) Density Which of the following would NOT be an extensive property? A.volume of a substance in beaker. B.mass of a soccer ball. C.boiling point of water. D.length of a class room.
What is the SI unit of mass? A) Meter B) Centimeter C) Gram D) Kilogram (kg) The symbol for density in SI system is: A. m3/s B. kg C. kg/m3 D. g/ml
Convert 240 K and 468 K to the Celsius scale. A) 513oC and 741oC B) -59oC and 351oC C) -18.3oC and 108oC D) -33oC and 295oC
Calculate the volume occupied by 4.50 X 102 g of gold (density = 19.3 g/cm3). A) 23.3 cm3 B) 8.69 x 103 cm3 C) 19.3 cm3 D) 450 cm3 The melting point of bromine is -7 oC. What is this melting point expressed in oF? A) 45 oF B) -28 oF C) -13 oF D) 19 oF
The number of significant figures in 0.053 is A. 3 B. 1 C. 4 D. 2 What is the Scientific form for 3,500,000,000 A. 3.5x109 B. 3x109 C. 3.5x108 D. 3.50x109
How many significant figures are there in the measurement 3.4080 g? A) 6 B) 5 C) 4 D) 3 E) 2 Multiply (4x 105) (6.2x107) 2.48 x 1013
Convert 357,096 to Scientific Notation 3.57096 x 105 Convert 0.005600 to Scientific Notation 5.600 x 10-3
Given: 2.46 x 106 + 3.476 x 103 Shift decimal 3 places to the left for 103. Move: 0.003476 x 103+3 Add: 2.46 x 106 + .003476 x 106 Answer: 2.463 x 106
Given: 5.762 x 103 2.65 x 10-1 Shift decimal 4 places to the right for 10-1. Move: 0.000265 x 10(-1+4) Subtract: 5.762 x 103-.000265 x 103 Answer: 5.762 x 103
6 5.4 x 10 Evaluate: 2 9.0 x 10 5.4 9.0 = 0.6 1. Divide the coefficients 6 10 2 = 10 4 10 2. Divide the powers of ten 4 0.6 x 10 3. Combine those results 3 6.0 x 10 4. Put in proper form
Practice Rule #1 Zeros 6 All digits count 45.8736 3 Leading 0 s don t .000239 5 .00023900 Trailing 0 s do 48000. 5 0 s count in decimal form 48000 3.982 106 2 0 s don t count w/o decimal 4 All digits count 1.00040 6 0 s between digits count as well as trailing in decimal form
Addition and Subtraction Look for the last important digit .71 __ ___ __ .56 + .153 = .713 82000 82000 + 5.32 = 82005.32 .1 10.0 - 9.8742 = .12580 0 10 9.8742 = .12580
Multiplication and division 32.27 1.54 = 49.6958 49.7 3.68 .07925 = 46.4353312 46.4 1.750 .0342000 = 0.05985 .05985 1.586 107 3.2650 106 4.858 = 1.586137 107 6.022 1023 1.661 10-24 = 1.000000 1.000