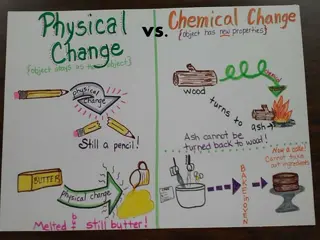Physical and Chemical Changes in Substances
Explore the differences between physical and chemical properties of substances, including observations that do not change the substance or mixture and those that result in something new. Learn to identify signs of a chemical change and remember them with handy cheat sheets. Understand how physical changes may alter the size, shape, or phase of a substance without changing its fundamental nature.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Physical Properties Observations that DO NOT CHANGE the substance or mixture Smell Touch Measure Hear See Taste
Chemical Properties Describes how the substance or mixture will react with another Combustibility Flammability Ability to React These observations CHANGE it into something new
Physical Changes and Chemical Changes
Signs of a Chemical Change Cheat Sheet! Precipitant is formed (liquids make a Solid) Energy change (Temperature or Light) Color change - Smell is produced Bubbles (gas) released -
TO HELP YOU REMEMBER: Chemical Change Cheat Sheet! Please Precipitant Excuse Energy change Coughs Color change Sneezes and Smell produced Burps Bubbles, gas produced
Signs of a Physical Change It can change size or shape (cut) (dissolve) It can change phases BUT IT IS STILL THE SAME!!!