Chemical Formulas and Subscripts in Chemistry
Chemical formulas and subscripts are fundamental concepts in chemistry for expressing the composition of substances at the molecular level. This article explores the significance of chemical formulas in representing elements and compounds like water (H2O), carbon dioxide (CO2), sodium chloride (NaCl), magnesium sulfate (MgSO4), and glucose. Additionally, it delves into the role of subscripts in indicating the number of atoms within a molecule and explains exceptions like those seen in compound formulas with parentheses. Dive into the world of chemistry and unravel the language of molecules!
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
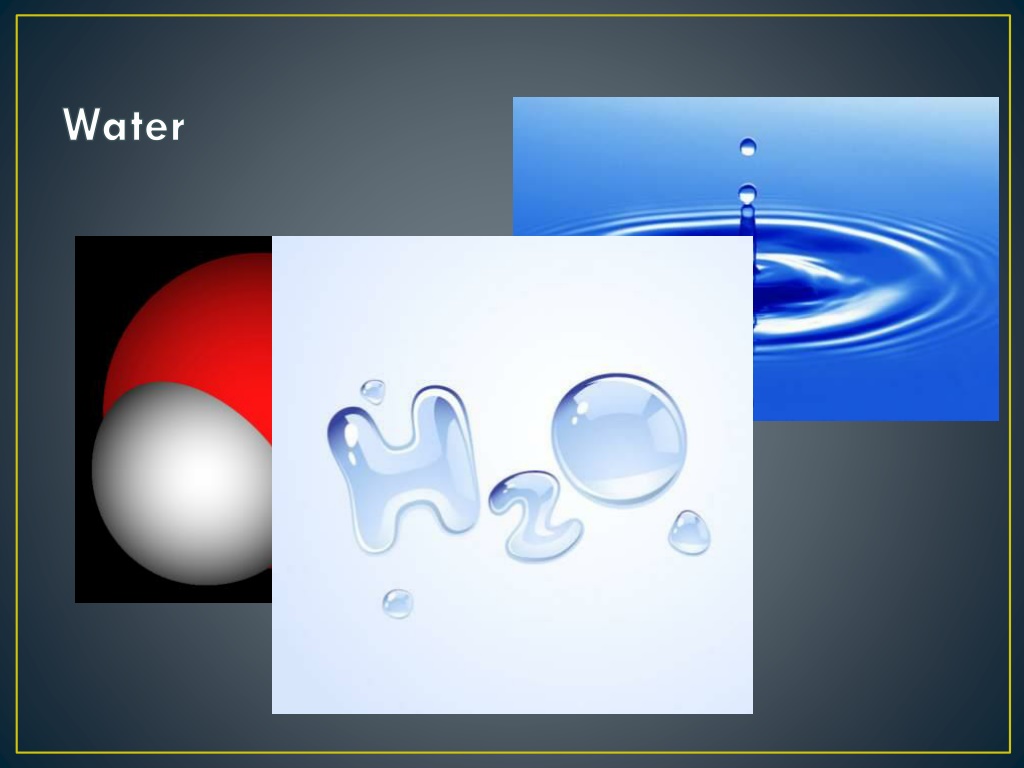
What about molecules? We have been studying about elements just by themselves such as oxygen and hydrogen. If you put them together you have a molecule of water (H2O). A cup of water contains millions of molecules of H2O.
What is a chemical formula? Chemical, or molecular, formulas are a concise way of expressing information about the atoms that constitute a particular chemical compound. Wait what? It is an expression which states the number and type of atoms present in a molecule of a substance.
Do you know what the chemical formulas are for the following substances?
For something more complicated. Glucose or sugar
Definition A subscript is used to represent the number of each atom being represented. If only one atom is represented, there is no subscript. In the formula for water, what is the subscript? There is only one atom of Oxygen, so it does not have a subscript. H2O H2O H = Hydrogen O = Oxygen
Some exceptions exist Usually the subscript just multiplies or shows the number of atoms of a single element. If the subscript exists outside of a set of parenthesis then it will multiply the atoms of all of the elements inside the parenthesis. N(CH3)3 How many of each atom are there now? Answer: Nitrogen-1, Carbon-3, Hydrogen-9 N = Nitrogen C = Carbon H = Hydrogen
In this image the Carbon atom is blue and the Oxygen atom is red. Write the chemical formula for this molecule. Answer: CO This substance is carbon monoxide
In this image the Carbon atom is black and the Oxygen atoms are red. Write the chemical formula for this molecule. Answer: CO3 This substance is carbonate
In this image Hydrogen atoms are white and Nitrogen atom is red. Write the chemical formula for this molecule. Answer: NH3 This substance is ammonia
In this image blue represents Nitrogen atoms, red represents Oxygen atoms, white represents Hydrogen atoms and black represents Carbon atoms. Write the chemical formula for this molecule. Answer: C8H10N4O2 This substance is caffeine
Definition Coefficients appear on the left side of a chemical formula. They are used to multiply all the atoms in a compound In the following formula, which is the coefficient? Earlier we learned that the subscript 2 meant that there were two Hydrogen atoms. The coefficient 7 means there are 7 times more. How many Hydrogen atoms do we have? How many Oxygen atoms? 7H2O 7H2O
Chemical Equations representation of chemical reaction in equation: a representation, using chemical symbols in a form resembling a mathematical equation, of the process involved in a chemical reaction 2H2+O2 2H2O This is an example of a chemical equation. The components on the left combine together to yield (represented by the arrow) the component on the right.
Yields 2H2+O2 2H2O Reactant
Atoms are neither created, nor destroyed, during any chemical reaction. This means that the same number of atoms that are present after a reaction are the same number of atoms that are present before a reaction. There is only a rearrangement
Subscripts are never changed when balancing an equation
Step 1 Write out your un-balanced equation using formulas of reactants and products. CH4+O2 CO2+H2O
Step 2 Count up the atoms in the products and reactants. How many carbons, hydrogens, and oxygens are on each side? Are they equal? CH4+O2 CO2+H2O C=1 H=2 O=3 C=1 H=4 O=2 They are NOT equal
Step 3 Since our carbons are ok we will not mess with those now. However, we have half the number of hydrogens in the products than we do in the reactants. What do we need to add? Where do we add it? CH4+O2 CO2+H2O CH4+O2 CO2+2H2O C=1 H=4 O=2 C=1 H=4 O=4 They are still NOT equal
Step 4 Now we have half the number of Oxygens. What do we need to add? Where do we need to add it? Is everything equal now? CH4+O2 CO2+2H2O CH4+2O2 CO2+2H2O C=1 H=4 O=4 C=1 H=4 O=4 They ARE now balanced
H2+O2H2O 2H2+O2 2H2O
Fe+Cl2FeCl3 2Fe+3Cl2 2FeCl3
Cu+AgNO3Cu(NO3)2+Ag Cu+2AgNO3 Cu(NO3)2+2Ag
Zn+HClZnCl2+H2 Zn+2HCl ZnCl2+H2
Pb(NO3)2+AlCl3PbCl2+Al(NO3)3 3Pb(NO3)2+2AlCl3 3PbCl2+2Al(NO3)3