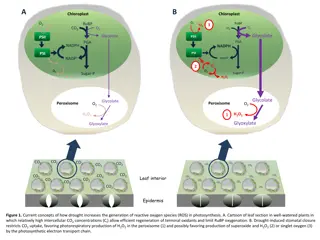
Impact of Bayesian Analysis in Preclinical Research
Explore the utilization of Bayesian analysis with informative priors in preclinical research to demonstrate statistical impact, featuring strategies, selling points, and contrasts with clinical settings. The use of Bayesian methodology allows for more efficient data analysis, reducing costs and resources while enabling more relevant and flexible modeling approaches.
Uploaded on | 0 Views
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Demonstrating the impact of statistics in the preclinical area using Bayesian analysis with informative priors Ros Walley, John Sherington, Joe Rastrick, Gill Watt Non Clinical Statistics Conference 8th-10thOctober 2014
2 Outline Strategy to demonstrate impact Features of Bayesian designs Selling points Preclinical setting Contrast with Clinical Related issues Case study First steps Types of control groups Bayesian methodology Summary to date
3 Strategy to demonstrate impact Features of Bayesian designs Explicit way of combining information sources Forces early agreement as to relevance of all information sources Reduce costs and resources (animal numbers) through informative priors/predictive distributions Reduce costs and resources through interim analysis Allows more relevant statements to be made at the end of the study e.g. the probability the response rate for drug A is more than 10% better than drug B Ranking compounds Comparing a combination with its components Flexibility in estimation. E.g. one can analyse on the log scale and estimate differences on the linear scale Allows for a wide variety of models to be fitted and can address issues such as lack of convergence or outlier-prone data Model averaging, model selection
4 Strategy to demonstrate impact Selling points of Bayesian methods High impact Focus on in vivo studies that are run again and again Saving even a few animals per study results in large savings, easily demonstrated Ground-breaking Quick search in the literature suggests little use in vivo except some focused applications: PK & PK/PD models SNPS/genes pathway analysis. Including a Nature reviews article called The Bayesian revolution in genetics Lookup proteins Complements clinical strategy *
5 Preclinical setting Contrast with clinical Preclinical Clinical Focus on individual study Hard to pool Standardized, locatable Easy to pool Data recording Software Graphpad PRISM, EXCEL SAS, R Relevant data for priors In house, Run to same protocol; any changes noted. Fantastic source. Usually from publications. Differences between protocols & populations. Summary level data only. Typically, N=20-100 per group for a PoC. Size of experiment For in vivo studies, typically N=3-10 per group Protocol finalisation A set of experiments may be further optimised during their running Very traditional approaches used. Little stats support. Reluctance to trigger a protocol amendment Novel stats methods Bayesian and adaptive methods implemented and published. Data interpretation Common to compare compounds across experiments Focus on individual experimental compound
Preclinical setting Related issues to consider Different output: internally & externally Getting hold of the data; in the right format Bayesian designs for repeated studies Rely entirely on historic data for one group Allied designs: modelled approach, QC charts Supplement a group with prior info analysis issues
7 Case study Background Mouse model to study inflammatory response after a challenge. Measures a number of cytokines at 2.5 or 3hrs. Includes test compounds + three control treatments: Negative group (no challenge). Positive group (challenge, no treatment). Comparator with known efficacy Main comparisons of interest: Test compound vs. +ve group (untreated) Does test compound reduce the response? Data on 19 studies available (reasonably consistent protocol). Most frequent in-vivo assay in this therapeutic area. Data typically presented an experiment at a time, in PRISM
8 First steps Strategy to smooth the introduction of Bayesian methods QC charts to demonstrate reproducibility over time Promoting interval estimates rather than a focus on p-values Before committing to reducing animals, show what would have happened if we had done this in the last study by removing observations from the data set Advertising and speaking to stakeholders Publication strategy: 1. A statistical paper case studies with details removed 2. For each assay, publish the meta-analysis of the historic data and the planned future data analysis preferably in the selected journal to be used for data for new compounds 3. Publish data relating to specific compounds having de-risked by the preceding 2 publications
9 First steps QC charts for control groups Well received. Introduces the idea of expt-to-expt variation. Intuitively, the relevance of the historic controls depends on the size of the study to study variation. Low expt-to-expt variation High expt-to-expt variation Bayesian analysis can use the historic control information, down-weighting it according to the amount of experiment-to-experiment variation
10 Types of control groups Not used for formal statistical comparisons. Example uses: To ensure challenge is working; to establish a window ; to check consistency with previous studies; to convert values to %. Replace group with a range from a predictive distribution Used for formal comparison vs. test compounds/doses Used as the comparison in t-tests ..etc Combine down-weighted historic data with the current experiment
11 Bayesian methodology Outline Analyse historic control treatment group data, excluding the last study. Bayesian meta-analysis Analyse the last study Show what would have happened if we had bought into the Bayesian approach; omit animals if necessary Possible options for future studies: Omit all/some animals from all/some control groups. Use historic data as prior information combined with observed data in a Bayesian analysis. Use historic data to give a predictive distribution for control group. i.e. don t include that treatment group in current study. Statistical model based on meta-analytic predictive methodology in Neuenschwander et al
12 Bayesian meta-analytic predictive approach Statistical model based on Neuenschwander et al (2010) Historic data:, h=1 H: Observed study data: Yh | h, a2~ N( h, a2) Study means: 1 H~ N( , 2) Predictions for next study, denoted by *: prior for next study True study mean * ~ N( , 2) predictive dn for next study Observed study mean Y*| *, a2~ N( *, a2/n*) Priors and hyperpriors has vague normal prior has vague half normal prior (sensitivity analysis carried out) a2has vague gamma prior
13 Bayesian methodology (2) Replacing control groups with predictive distributions Bayesian analysis of historic control data New study data: 8 per group (for the other groups) QC-chart like limits for control group Traditional analysis of current study Overlay Bayesian analysis in data presentations Suitable for control groups not used in statistical comparisons
14 Case study Predictive distribution
15 Bayesian methodology (4) Full Bayesian analysis Bayesian analysis of historic control data New study data: 8 per group Effectively N control animals with mean, m Bayesian analysis of current study Suitable for any control groups but requires a Bayesian analysis for each data set. Results and conclusions This is a simplification of the exact analysis
16 Case Study Full Bayesian analysis The Bayesian analysis gives narrower confidence intervals. It is comparable to using 3 extra animals (11 instead of 8) __________ A@3mg/kg ___________ A@30mg/kg __________ B@3mg/kg ___________ B@30mg/kg
17 Summary to date Predictive approach developed for two models Biologists very positive; implementation being considered Potential saving: one control group per study Full Bayesian approach developed for one model Modest savings in numbers of animals Software issue; potentially lengthening turnaround times for rapid screens Biologists suggested starting with something slightly more low-key QC charts provided an excellent introduction to between and within study variation Bayesian methods can reduce required resource (animal numbers) To have the greatest impact, focus on studies repeated very frequently Even for well controlled in vivo experiments study-to-study variation is not negligible ,
18 References Neuenschwander, B., Capkun-Niggli, G., Branson, M. and Spiegelhalter, DJ. Summarizing historical information on controls in clinical trials., Clin Trials 2010 7: 5 Di Scala L, Kerman J, Neuenschwander B. Collection, synthesis, an interpretation of evidence: a proof-of-concept study in COPD. Statistics in Medicine 2013; 32: 1621 1634. Evans, M, Hastings, N and Peacock, B (2000). Statistical Distributions. 3rd Edition. New York: Wiley Beaumont and Rannala, The Bayesian revolution in genetics. Nature Reviews Genetics 5, 251-261 (April 2004)
19 Questions?