GCSE Separation Challenge: Iron, Sulfur, Sand, and Food Dyes Mixture
Students are tasked with separating a mixture containing iron, sulfur, sand, and food dyes using various techniques. They work in pairs, following provided instructions and using specific equipment. Marks are awarded based on successful separation and organization. The challenge involves planning, executing the separation process, and creating a clear chromatogram. www.thescienceteacher.co.uk.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
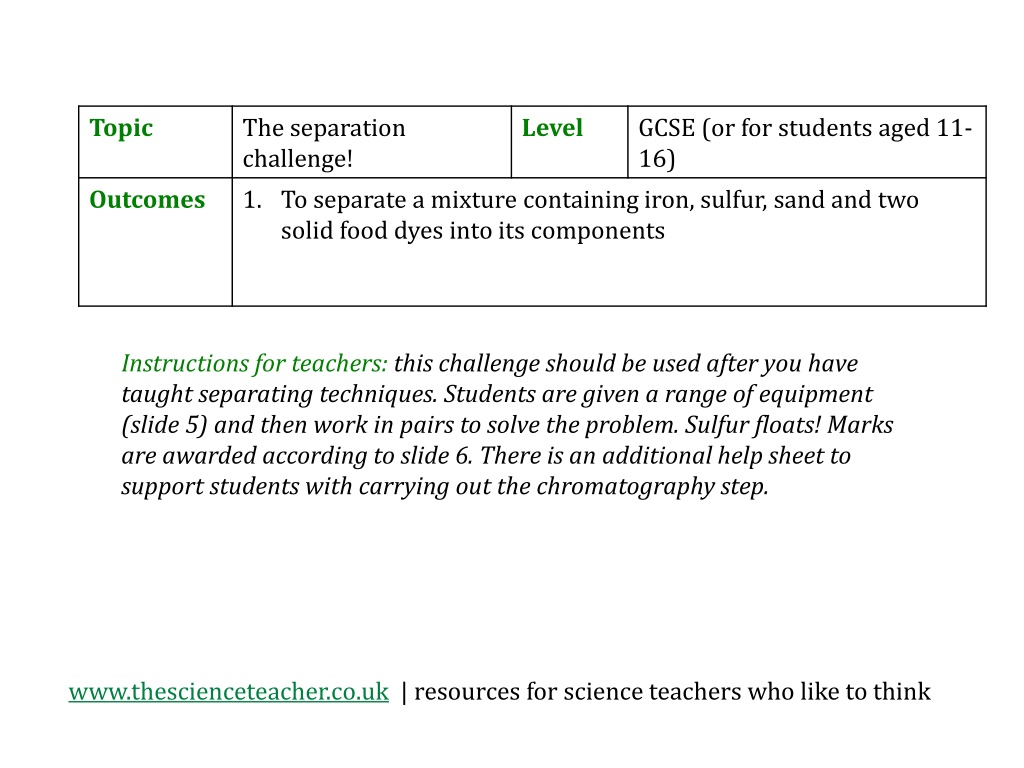
Topic The separation challenge! 1. To separate a mixture containing iron, sulfur, sand and two solid food dyes into its components Level GCSE (or for students aged 11- 16) Outcomes Instructions for teachers: this challenge should be used after you have taught separating techniques. Students are given a range of equipment (slide 5) and then work in pairs to solve the problem. Sulfur floats! Marks are awarded according to slide 6. There is an additional help sheet to support students with carrying out the chromatography step. www.thescienceteacher.co.uk | resources for science teachers who like to think
Separation Challenge!
Oops, I have a problem How many substances are in my mixture?
Your mission: Separate the mixture into its ? different substances. Iron filings Sulfur Sand Food colourings ?
You will be given: Plastic freezer bag (small) Magnet Filter paper Filter funnel Spatula Stirring rod 250 ml beaker x 2 Tongs 100 ml water in a wash bottle Magnifying glass Splint Paperclip Chromatography paper cut to size 3 small weighing boats Marker pen Measuring cylinder 50 ml
At the end of the challenge you should have: The iron, sand and sulfur in three plastic pots labelled with your names (2 marks) Have a clear chromatogram (4 marks) A tidy desk at the end (2 marks) We will weigh the mass of iron filings you have separated (4 marks)
5 minutes! Thinking and planning time: How will you do the separation? In what order should you do the separation? Which equipment will you use? How will you do the chromatography? Top Tip! plan out what you are going to do on a piece of rough paper
20 minutes Get separating! Iron filings Sulfur Sand Food colourings